Chemical Constituents from Stem Bark and Roots of Clausena anisata
Abstract
:1. Introduction
2. Results and Discussion
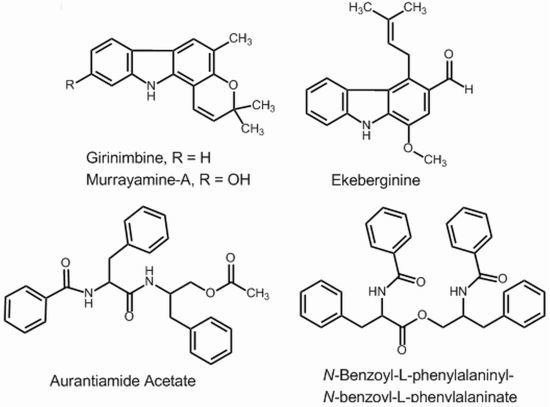
2.1. Isolation and NMR (1H and 13C) Analysis
2.2. Mass Spectrometry of the Isolated Compounds
2.3. Biosynthesis and Biological Activity
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
4. Conclusions
Acknowledgments
References
- Letouzey, R. Flore du Gabon, Volume 6: Rutaceae, Zygophyllaceae, Balanitaceae; Muséum National d’Histoire Naturelle: Paris, France, 1963. [Google Scholar]
- Hamza, O.J.M.; van den Bout-van den Beukel, C.J.P.; Matee, M.I.N.; Moshi, M.J.; Mikx, F.H.M.; Selemani, H.O.; Mbwambo, Z.H.; Van der Ven, A.J.A.M.; Verweij, P.E. Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J. Ethnopharmacol. 2006, 108, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Moshi, M.J.; Kagashe, G.A.B.; Mbwambo, Z.H. Plants used to treat epilepsy by Tanazanian traditional healers. J. Ethnopharmacol. 2005, 97, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Uwaifo, A.O. The mutagenicities of seven coumarin derivatives and a furan derivative (nimbolide) isolated from three medicinal plants. J. Toxicol. Environ. Health 1984, 13, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, W.; Ritthiwigrom, T.; Cheenpracha, S.; Promgool, T.; Yossathera, K.; Deachathai, S.; Phakhodee, W.; Laphookhieo, S. Bioactive carbazole alkaloids from Clausena wallichii roots. J. Nat. Prod. 2012, 75, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Songsiang, U.; Thongthoom, T.; Boonyarat, C.; Yenjai, C. Claurailas A–D, cytotoxic carbazole alkaloids from the roots of Clausena harmandiana. J. Nat. Prod. 2011, 74, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Chowdhury, B.K.; Bhattacharyya, P. Clausenol and clausenine—two carbazole alkaloids from Clausena anisata. Phytochemistry 1995, 40, 295–298. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Aizawa, K.; Yoshida, K.; Ruangrungsi, N.; Furukawa, H. γ-Lactone carbazoles from Clausena anisata. J. Nat. Prod. 2009, 72, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Katsuno, S.; Itoigawa, M.; Ruangrungsi, N.; Mukainaka, T.; Okuda, M.; Kitagawa, Y.; Tokuda, H.; Nishino, H.; Furukawa, H. New carbazole alkaloids from Clausena anisata with antitumor promoting activity. J. Nat. Prod. 2000, 63, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Ngadjui, B.T.; Ayafor, J.F.; Sondengam, B.L.; Connolly, J.D. Quinoline and carbazole alkaloids from Clausena anisata. Phytochemistry 1989, 28, 1517–1519. [Google Scholar] [CrossRef]
- Okorie, D.A. New carbazole alkaloids and coumarins from roots of Clausena anisata. Phytochemistry 1975, 14, 2720–2721. [Google Scholar] [CrossRef]
- Joshi, B.S.; Kamat, V.N.; Saksena, A.K.; Govindachari, T.R. Structure of heptaphylline, a carbazole alkaloid from Clausena heptaphylla Wt. & Arn. Tetrahedron Lett. 1967, 8, 4019–4022. [Google Scholar]
- Lakshmi, V.; Raj, D.P.K.; Kapil, R.S.; Popli, S.P. Monoterpenoid furanocoumarin lactones from Clausena anisata. Phytochemistry 1984, 23, 2629–2631. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Mouncherou, S.M.; Ayafor, J.F.; Sondengam, B.L.; Tillequin, F. Geranyl coumarins from Clausena anisata. Phytochemistry 1991, 30, 2809–2811. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Ayafor, J.F.; Sondengam, B.L.; Connolly, J.D. Coumarins from Clausena anisata. Phytochemistry 1989, 28, 585–589. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Ayafor, J.F.; Sondengam, B.L.; Connolly, J.D. Prenylated coumarins from the leaves of Clausena anisata. J. Nat. Prod. 1989, 52, 243–247. [Google Scholar] [CrossRef]
- Ngadjui, B.T.; Ayafor, J.F.; Sondengam, B.L.; Connolly, J.D. Limonoids from Clausena anisata. J. Nat. Prod. 1989, 52, 832–836. [Google Scholar] [CrossRef]
- Meva’a, L.M.; Songue, J.L.; Wansi, J.D.; Waffo, A.F.K.; Dongo, E.; Mpondo, T.N.; Sewald, N. Acridone alkaloids and coumarins from the stem bark of Citropsis articulata (Rutaceae). Z. Naturforsch. 2010, 65b, 525–527. [Google Scholar] [CrossRef]
- Bakar, N.H.A.; Sukari, M.A.; Rahmani, M.; Sharif, A.M.; Khalid, K.; Yusuf, U.K. Chemical constituents from stem bark and roots of Murraya koenigii (Rutaceae). Mal. J. Anal. Sci. 2007, 11, 173–176. [Google Scholar]
- Joshi, B.S.; Kamat, V.N.; Gawad, D.H. On structures of girinimbine, mahanimbine, isomahanimbine, koenimbidine and murrayacine. Tetrahedron 1970, 26, 1475–1482. [Google Scholar] [CrossRef]
- Furukawa, H.; Wu, T.S.; Ohta, T.; Kuoh, C.S. Chemical constituents of Murraya euchrestifolia HAYATA. Structures of novel carbazolequinones and other new carbazole alkaloids. Chem. Pharm. Bull. 1985, 33, 4132–4138. [Google Scholar] [CrossRef]
- Wu, T.S. Murrayamine-A, murrayamine-B, murrayamine-C and (+)-mahanine, carbazole alkaloids from Murraya euchrestifolia. Phytochemistry 1991, 30, 1048–1051. [Google Scholar] [CrossRef]
- Begum, R.; Rahman, M.S.; Chowdhury, A.M.S.; Rahman, M.M.; Rashid, M.A. O-methylheptaphylline from Clausena suffruticosa. Nat. Prod. Commun. 2008, 3, 815–818. [Google Scholar]
- Lontsi, D.; Ayafor, J.F.; Sondengam, B.L.; Connolly, J.D.; Rycroft, D.S. The use of two-dimensional long-range δC/δH correlation in conjunction with the one-dimensional proton-coupled 13C NMR spectrum in the structural elucidation of ekeberginine, a new carbazole alkaloid from Ekebergia senegalensis (Meliaceae). Tetrahedron Lett. 1985, 26, 4249–4252. [Google Scholar] [CrossRef]
- Ishiguro, K.; Nagata, S.; Fukumoto, H.; Yamaki, M.; Takagi, S.; Isoi, S. A dipeptide derivative from Hypericum japonicum. Phytochemistry 1991, 30, 3639–3641. [Google Scholar] [CrossRef]
- Isshiki, K.; Asai, Y.; Tanaka, S.; Nishio, M.; Uchida, T.; Okuda, T.; Komatsubara, S.; Sakurai, N. Aurantiamide acetate, a selective cathepsin inhibitor, produced by Aspergillus penicilloides. Biosci. Biotechnol. Biochem. 2001, 65, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.C.; Thomson, R.H. A modified dipeptide from the alga Cystoseira corniculata Hauck. Experientia 1976, 32, 1106–1107. [Google Scholar] [CrossRef]
- Wahidulla, S.; D’Souza, L.; Kamat, S.Y. Dipeptides from the red alga Acanthospora spicifera. Phytochemistry 1991, 30, 3323–3325. [Google Scholar] [CrossRef]
- Uemura, D.; Sugiura, K.; Hirata, Y. O-Acetyl-N-(N-benzoyl-l-phenylalanyl)-l-phenylalaninol. Isolation from Euphorbia fischeriana. Chem. Lett. 1975, 537–538. [Google Scholar] [CrossRef]
- Catalán, C.A.N.; de Heluani, C.S.; Kotowicz, C.; Gedris, T.E.; Herz, W. A linear sesterterpene, two squalene derivatives and two peptide derivatives from Croton hieronymi. Phytochemistry 2003, 64, 625–629. [Google Scholar] [CrossRef]
- Banerji, A.; Das, R. Aurantiamide and aurantiamide acetate, new amides from Piper aurantiacum Wall. Ind. J. Chem. 1975, 13, 1234–1236. [Google Scholar]
- Banerji, A.; Ray, R. Aurantiamides, a new class of modified dipeptides from Piper aurantiacum. Phytochemistry 1981, 20, 2217–2220. [Google Scholar] [CrossRef]
- Poi, R.; Adityachoudhury, N. Occurrence of two rare amides in Medicago polymorpha. Indian J. Chem. Sect. B 1986, 25, 1245–1246. [Google Scholar]
- Talapatra, S.K.; Mallik, A.K.; Talapatra, B. Pongaglabol, a new hydroxyfuranoflavone, and aurantiamide acetate, a dipeptide from the flowers of Pongamia glabra. Phytochemistry 1980, 19, 1199–1202. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Raju, S.N. Chemical constituents of the bark and leaves of Pterospermum heyneanum Wall. J. Indian Chem. Soc. 1988, 65, 147–148. [Google Scholar]
- Sashidhara, K.V.; Rosaiah, J.N.; Tyagi, E.; Shukla, R.; Raghubir, R.; Rajendran, S.M. Rare dipeptide and urea derivatives from roots of Moringa oleifera as potential anti-inflammatory and antinociceptive agents. Eur. J. Med. Chem. 2009, 44, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Castro, V.; Povera, L.; Chavarria, M.; Murillo, R. Chemical constituents from Zanthoxylum setulosum (Rutaceae). Boletin Latinoamricano y del Caribe de Plantas Medicinales y Aromaticas 2011, 10, 155–158. [Google Scholar]
- Clark, A.M.; Hufford, C.D.; Robertson, L.W. Two metabolites from Aspergillus flavipes. J. Nat. Prod. 1977, 40, 146–151. [Google Scholar]
- Talapatra, S.K.; Pal, M.K.; Mallik, A.K.; Talapatra, B. Structure and synthesis of (−)-anabellamide. A new phenylalanine derived ester amide from Anaphalis subumbellata: occurrence of 4′-hydroxydehydrokawain. J. Nat. Prod. 1983, 46, 140–143. [Google Scholar]
- McCorkindale, N.J.; Baxter, R.L.; Roy, T.P.; Shields, H.S.; Stewart, R.M.; Hutchinson, S.A. Synthesis and chemistry of N-benzoyl-O-[N′-benzoyl-l-phenylalanyl]-l-phenylalaninol, the major mycelia metabolite of Penicillum canadense. Tetrahedron 1978, 34, 2791–2795. [Google Scholar] [CrossRef]
- Bird, B.A.; Campbell, I.M. Disposition of mycophenolic acid, brevianamide A, asperphenamate, and ergosterol in solid cultures of Penicillum brevicompactum. Appl. Environ. Microbiol. 1982, 4, 345–348. [Google Scholar]
- Nozawa, K.; Udagawad, S.I.; Nakajima, S.; Kawai, K.-I. A dioxopiperazine derivative from Penicillum megasporum. Phytochemistry 1989, 28, 929–931. [Google Scholar] [CrossRef]
- Ferreira, D.T.; Silva, R.B.; Deoliveira, A.B.; Isobe, M.; Braz, R. Dipeptide from the roots of Zeyhera digitalis. J. Brazil. Chem. Soc. 1995, 6, 323–326. [Google Scholar] [CrossRef]
- de Carvalho, M.G.; Cardozo, M.A.R.; Catunda, F.E.A., Jr.; de Carvalho, A.G. The chemical constituents from Piptadenia gonoacantha (Mart.) J.F. Macbr (pau jacaré). Ann. Brazil. Acad. Sci. 2010, 82, 561–567. [Google Scholar] [CrossRef]
- Hashim, N.M.; Rahmani, M.; Shamaun, S.S.; Ee, G.C.L.; Sukari, M.A.; Ali, A.M.; Go, R. Dipeptide and xanthones from Artocarpus kemando Miq. J. Med. Plants Res. 2011, 5, 4224–4230. [Google Scholar]
- Nielsen, K.F.; Månsson, M.; Rank, C.; Frisvad, J.C.; Larsen, T.O. Dereplication of microbial natural products by LC-DAD-TOFMS. J. Nat. Prod. 2011, 74, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 15 November 2012).
- Grossert, J.S.; Cook, M.C.; White, R.L. The influence of structural features on facile McLafferty-type, even-electron rearrangements in tandem mass spectra of carboxylate anions. Rapid Commun. Mass Spectrom. 2006, 20, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.W.; Reddy, K.R.; Knölker, H.-J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012, 112, 3193–3328. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, Y.; Lin, S.; Li, C.; Zhou, C.; Wang, S.; Huang, H.; Liu, P.; Ye, G.; Shen, X. Induction of cell cycle arrest by the carbazole alkaloid Clauszoline-I from Clausena vestita D. D. Tao via inhibition of the PKCδ phosphorylation. Eur. J. Med. Chem. 2012, 47, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kok, Y.Y.; Mooi, L.Y.; Ahmad, K.; Sukari, M.A.; Mat, N.; Rahmani, M.; Ali, A.M. Anti-tumour promoting activity and antioxidant properties of girinimbine isolated from the stem bark of Murraya koenigii S. Molecules 2012, 17, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Abdul, A.B.; Sukari, M.A.; Mohan, S.; Abdelwahab, S.I.; Wah, T.S. The growth suppressing effects of girinimbine on Hepg2 involve induction of apoptosis and cell cycle arrest. Molecules 2011, 16, 7155–7170. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-B.; Yan, S.-Y.; Cai, B.; Yao, X.-S. Carbazole alkaloids as new cell cycle inhibitor and apoptosis inducers from Clausena dunniana levl. J. Asian Nat. Prod. Res. 2002, 4, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Li, Y.; Zou, C.; Wang, C.; Gao, J.; Miao, C.; Ma, E.; Sun, T. Synthesis and in vitro antitumor activity of asperphenamate derivatives as autophagy inducer. Bioorg. Med. Chem. Lett. 2012, 22, 2216–2220. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wang, J.H.; Sun, T.M. Total synthesis and anticancer activity studies of the stereoisomers of asperphenamate and patriscabratine. Chin. Chem. Lett. 2010, 21, 155–158. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gray, A.I. A benzoisofuranone derivative and carbazole alkaloids from Murray koenigii and their antimicrobial activity. Phytochemistry 2005, 66, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Adebajo, A.C.; Ayoola, O.F.; Iwalewa, E.O.; Akindahunsi, A.A.; Omisore, N.O.A.; Adewunmi, C.O.; Adenowo, T.K. Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine 2006, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.-N.; Lee, Y.-S.; Wu, T.-S.; Teng, C.-M. Inhibition of cyclooxygenase activity and increase in platelet cyclic-AMP by girinimbine, isolated from Murraya euchrestifolia. Biochem. Pharmacol. 1994, 48, 353–360. [Google Scholar] [PubMed]
- Wu, T.-S.; Chan, Y.-Y.; Liou, M.-J.; Lin, F.-W.; Shi, L.-S.; Chen, K.-T. Platelet aggregation inhibitor from Murraya euchrestifolia. Phytother. Res. 1998, 12, S80–S82. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Hwang, T.-L.; Lin, Y.-T.; Kuo, Y.-C.; Leu, Y.-L. Chemical constituents from Lobelia chinensis and their anti-virus and anti-inflammatory bioactivities. Arch. Pharm. Res. 2011, 34, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Wang, Y.; Zhang, Y. Bioassay-guided screening and isolation of α-glucosidase and tyrosinase inhibitors from leaves of Morus alba. Food Chem. 2012, 131, 617–625. [Google Scholar] [CrossRef]
- Balunas, M.J.; Su, B.; Riswan, S.; Fong, H.H.S.; Brueggemeier, R.W.; Pezzuto, J.M.; Kinghorn, A.D. Isolation and characterization of aromatase inhibitors from Brassaiopsis glomerulata (Araliaceae). Phytochem. Lett. 2009, 2, 29–33. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




© 2012 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Songue, J.L.; Kouam; Dongo, E.; Mpondo, T.N.; White, R.L. Chemical Constituents from Stem Bark and Roots of Clausena anisata. Molecules 2012, 17, 13673-13686. https://doi.org/10.3390/molecules171113673
Songue JL, Kouam, Dongo E, Mpondo TN, White RL. Chemical Constituents from Stem Bark and Roots of Clausena anisata. Molecules. 2012; 17(11):13673-13686. https://doi.org/10.3390/molecules171113673
Chicago/Turabian StyleSongue, Jules Lobe, Kouam, Etienne Dongo, Theophile Ngando Mpondo, and Robert L. White. 2012. "Chemical Constituents from Stem Bark and Roots of Clausena anisata" Molecules 17, no. 11: 13673-13686. https://doi.org/10.3390/molecules171113673
APA StyleSongue, J. L., Kouam, Dongo, E., Mpondo, T. N., & White, R. L. (2012). Chemical Constituents from Stem Bark and Roots of Clausena anisata. Molecules, 17(11), 13673-13686. https://doi.org/10.3390/molecules171113673