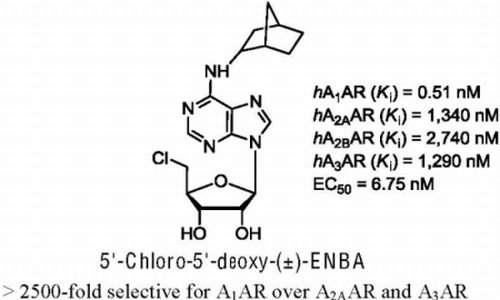
5'-Chloro-5'-deoxy-(±)-ENBA, a Potent and Selective Adenosine A1 Receptor Agonist, Alleviates Neuropathic Pain in Mice Through Functional Glial and Microglial Changes without Affecting Motor or Cardiovascular Functions
Abstract
:1. Introduction
2. Results
2.1. 5′Cl5′d-(±)-ENBA Reduced Thermal Hyperalgesia and Mechanical Allodynia in SNI Mice and Did Not Affect Motor or Cardiovascular Functions



| Sham + Vehicle | Sham + 5′Cl5'd-(±)-ENBA | SNI + Vehicle | SNI + 5′Cl5'd-(±)-ENBA | |
|---|---|---|---|---|
| Systolic Blood Pressure (mmHg) | 113 ± 2.4 | 111.3 ± 1.9 | 110.4 ± 2.1 | 108.4 ± 2.6 |
| Heart Rate (BPM) | 520.12 ± 3.9 | 518.12 ± 2.9 | 512.31 ± 3.4 | 515.15 ± 2.3 |
2.2. 5′Cl5′d-(±)-ENBA-Induced Analgesia is Associated with a Reduction in Glial and Microglial Activation 7 Days after SNI Induction


2.3. A1AR is over-Expressed in Glial Cells after Peripheral Nerve Injury

3. Discussion
4. Experimental
4.1. Animals
4.2. Spared Nerve Injury
4.3. Nociceptive Behaviour
4.4. Motor Coordination Behaviour
4.5. Non-Invasive Blood Pressure and Heart Rate Measurements
4.6. Spinal Cord Immunohistochemistry
4.7. Quantitative Image Analysis
4.8. Treatments
4.9. Drugs
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
References
- Bonica, J.J. Pain-basic principles of management. Northwest Med. 1970, 69, 567–568. [Google Scholar]
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Clark, A.K.; Wodarski, R.; Guida, F.; Sasso, O.; Malcangio, M. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia 2010, 58, 1710–1726. [Google Scholar] [CrossRef]
- Clark, A.K.; Yip, P.K.; Grist, J.; Gentry, C.; Staniland, A.A.; Marchand, F.; Dehvari, M.; Wotherspoon, G.; Winter, J.; Ullah, J.; et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 10655–10660. [Google Scholar]
- Clark, A.K.; Yip, P.K.; Malcangio, M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J. Neurosci. 2009, 29, 6945–6954. [Google Scholar] [CrossRef]
- Luongo, L.; Palazzo, E.; Tambaro, S.; Giordano, C.; Gatta, L.; Scafuro, M.A.; Rossi, F.S.; Lazzari, P.; Pani, L.; de Novellis, V.; et al. 1-(2',4'-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol. Dis. 2010, 37, 177–185. [Google Scholar] [CrossRef]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar]
- Haskó, G.; Szabó, C.; Németh, Z.H.; Kvetan, V.; Pastores, S.M.; Vizi, E.S. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 1996, 157, 4634–4640. [Google Scholar]
- Bura, S.A.; Nadal, X.; Ledent, C.; Maldonado, R.; Valverde, O. A2A adenosine receptor regulates glia proliferation and pain after peripheral nerve injury. Pain 2008, 140, 95–103. [Google Scholar] [CrossRef]
- Abo-Salem, O.M.; Hayallah, A.M.; Bilkei-Gorzo, A.; Filipek, B.; Zimmer, A.; Müller, C.E. Antinociceptive effects of novel A2B adenosine receptor antagonists. J. Pharmacol. Exp. Ther. 2004, 308, 358–366. [Google Scholar]
- Schenone, S.; Brullo, C.; Musumeci, F.; Bruno, O.; Botta, M. A1 receptors ligands: Past, present and future trends. Curr. Top. Med. Chem. 2010, 10, 878–901. [Google Scholar] [CrossRef]
- Franchetti, P.; Cappellacci, L.; Marchetti, S.; Trincavelli, L.; Martini, C.; Mazzoni, M.R.; Lucacchini, A.; Grifantini, M. 2'-C-Methyl analogues of selective adenosine receptor agonists: Synthesis and binding studies. J. Med. Chem. 1998, 41, 1708–1715. [Google Scholar] [CrossRef]
- Cappellacci, L.; Franchetti, P.; Pasqualini, M.; Petrelli, R.; Vita, P.; Lavecchia, A.; Novellino, E.; Costa, B.; Martini, C.; Klotz, K.N.; et al. Synthesis, biological evaluation and molecular modeling of ribose-modified adenosine analogues as adenosine receptors agonists. J. Med. Chem. 2005, 48, 1550–1562. [Google Scholar] [CrossRef]
- Cappellacci, L.; Franchetti, P.; Vita, P.; Petrelli, R.; Lavecchia, A.; Costa, B.; Spinetti, F.; Martini, C.; Klotz, K.N.; Grifantini, M. 5'-Carbamoyl derivatives of 2'-C-methyl-purine nucleosides as selective A1 adenosine receptor agonists: Affinity, efficacy, and selectivity for A1 receptor from different species. Bioorg. Med. Chem. 2008, 16, 336–353. [Google Scholar] [CrossRef]
- Franchetti, P.; Cappellacci, L.; Vita, P.; Petrelli, R.; Lavecchia, A.; Kachler, S.; Klotz, K.N.; Marabese, I.; Luongo, L.; Maione, S.; et al. N6-Cycloalkyl- and N6-bicycloalkyl-C5'(C2')-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice. J. Med. Chem. 2009, 52, 2393–2406. [Google Scholar]
- Sowa, N.A.; Taylor-Blake, B.; Zylka, M.J. Ecto-5'-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits. J. Neurosci. 2010, 30, 2235–2244. [Google Scholar] [CrossRef]
- Sowa, N.A.; Voss, M.K.; Zylka, M.J. Recombinant ecto-5'-nucleotidase (CD73) has long lasting antinociceptive effects that are dependent on adenosine A1 receptor activation. Mol. Pain 2010, 6, 2–8. [Google Scholar] [CrossRef]
- Maione, S.; de Novellis, V.; Cappellacci, L.; Palazzo, E.; Vita, D.; Luongo, L.; Stella, L.; Franchetti, P.; Marabese, I.; Rossi, F.; et al. The antinociceptive effect of 2-chloro-2'-C-methyl-N6-cyclopentyladenosine (2'-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain 2007, 131, 281–292. [Google Scholar] [CrossRef]
- Wu, W.P.; Hao, J.X.; Halldner, L.; Lövdahl, C.; DeLander, G.E.; Wiesenfeld-Hallin, Z.; Fredholm, B.B.; Xu, X.J. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain 2005, 113, 395–404. [Google Scholar] [CrossRef]
- Curros-Criado, M.M.; Herrero, J.F. The antinociceptive effects of the systemic adenosine A1 receptor agonist CPA in the absence and in the presence of spinal cord sensitization. Pharmacol. Biochem. Behav. 2005, 82, 721–726. [Google Scholar] [CrossRef]
- Stella, L.; Berrino, L.; Maione, S.; de Novellis, V.; Rossi, F. Cardiovascular effects of adenosine and its analogs in anaesthetized rats. Life Sci. 1993, 53, 755–763. [Google Scholar] [CrossRef]
- Schindler, C.W.; Karcz-Kubicha, M.; Thorndike, E.B.; Müller, C.E.; Tella, S.R.; Ferré, S.; Goldberg, S.R. Role of central and peripheral adenosine receptors in the cardiovascular responses to intraperitoneal injections of adenosine A1 and A2A subtype receptor agonists. Br. J. Pharmacol. 2005, 144, 642–650. [Google Scholar] [CrossRef]
- Tsutsui, S.; Schnermann, J.; Noorbakhsh, F.; Henry, S.; Yong, V.W.; Winston, B.W.; Warren, K.; Power, C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 2004, 24, 1521–1529. [Google Scholar] [CrossRef]
- Synowitz, M.; Glass, R.; Färber, K.; Markovic, D.; Kronenberg, G.; Herrmann, K.; Schnermann, J.; Nolte, C.; van Rooijen, N.; Kiwit, J.; et al. A1 adenosine receptors in microglia control glioblastoma-host interaction. Cancer Res. 2006, 66, 8550–8557. [Google Scholar]
- Johnston, J.B.; Silva, C.; Gonzalez, G.; Holden, J.; Warren, K.G.; Metz, L.M.; Power, C. Diminished adenosine A1 receptor expression on macrophages in brain and blood of patients with multiple sclerosis. Ann. Neurol. 2001, 49, 650–658. [Google Scholar] [CrossRef]
- Trussell, L.O.; Jackson, M.B. Adenosine-activated potassium conductance in cultured striatal neurons. Proc. Natl. Acad. Sci. USA 1985, 82, 4857–4861. [Google Scholar] [CrossRef]
- MacDonald, R.L.; Skerritt, J.H.; Werz, M.A. Adenosine agonists reduce voltage-dependent calcium conductance of mouse sensory neurones in cell culture. J. Physiol. 1986, 370, 75–90. [Google Scholar]
- Zylka, M.J. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol. Med. 2011, 17, 188–196. [Google Scholar] [CrossRef]
- Korboukh, I.; Hull-Ryde, E.A.; Rittiner, J.E.; Randhawa, A.S.; Coleman, J.; Fitzpatrick, B.J.; Setola, V.; Janzen, W.P.; Frye, S.V.; Zylka, M.J.; et al. Orally active adenosine A1 receptor agonists with antinociceptive effects in mice. J. Med. Chem. 2012, 14, 6467–6477. [Google Scholar]
- Shields, S.D.; Eckert, W.A., 3rd; Basbaum, A.I. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J. Pain 2003, 4, 465–470. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef]
- Fortin, A.; Harbour, D.; Fernandes, M.; Borgeat, P.; Bourgoin, S. Differential expression of adenosine receptors in human neutrophils: Up-regulation by specific Th1 cytokines and lipopolysaccharide. J. Leuk. Biol. 2006, 79, 574–585. [Google Scholar]
- Cronstein, B.N.; Naime, D.; Ostad, E. The antiinflammatory effects of methotrexate are mediated by adenosine. Adv. Exp. Med. Biol. 1994, 370, 411–416. [Google Scholar]
- Costigan, M.; Moss, A.; Latremoliere, A.; Johnston, C.; Verma-Gandhu, M.; Herbert, T.A.; Barrett, L.; Brenner, G.J.; Vardeh, D.; Woolf, C.J.; et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 2009, 29, 14415–14422. [Google Scholar]
- Beers, D.R.; Henkel, J.S.; Zhao, W.; Wang, J.; Appel, S.H. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA 2008, 105, 15558–15563. [Google Scholar]
- Moss, A.; Beggs, S.; Vega-Avelaira, D.; Costigan, M.; Hathway, G.J.; Salter, M.W.; Fitzgerald, M. Spinal microglia and neuropathic pain in young rats. Pain 2007, 128, 215–224. [Google Scholar] [CrossRef]
- Stanley, P.; Guidos, C.J. Regulation of Notch signaling during T- and B-cell development by O-fucose glycans. Immunol. Rev. 2009, 230, 201–215. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound 5′Cl5′d-(±)-ENBA are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luongo, L.; Petrelli, R.; Gatta, L.; Giordano, C.; Guida, F.; Vita, P.; Franchetti, P.; Grifantini, M.; Novellis, V.D.; Cappellacci, L.; et al. 5'-Chloro-5'-deoxy-(±)-ENBA, a Potent and Selective Adenosine A1 Receptor Agonist, Alleviates Neuropathic Pain in Mice Through Functional Glial and Microglial Changes without Affecting Motor or Cardiovascular Functions. Molecules 2012, 17, 13712-13726. https://doi.org/10.3390/molecules171213712
Luongo L, Petrelli R, Gatta L, Giordano C, Guida F, Vita P, Franchetti P, Grifantini M, Novellis VD, Cappellacci L, et al. 5'-Chloro-5'-deoxy-(±)-ENBA, a Potent and Selective Adenosine A1 Receptor Agonist, Alleviates Neuropathic Pain in Mice Through Functional Glial and Microglial Changes without Affecting Motor or Cardiovascular Functions. Molecules. 2012; 17(12):13712-13726. https://doi.org/10.3390/molecules171213712
Chicago/Turabian StyleLuongo, Livio, Riccardo Petrelli, Luisa Gatta, Catia Giordano, Francesca Guida, Patrizia Vita, Palmarisa Franchetti, Mario Grifantini, Vito De Novellis, Loredana Cappellacci, and et al. 2012. "5'-Chloro-5'-deoxy-(±)-ENBA, a Potent and Selective Adenosine A1 Receptor Agonist, Alleviates Neuropathic Pain in Mice Through Functional Glial and Microglial Changes without Affecting Motor or Cardiovascular Functions" Molecules 17, no. 12: 13712-13726. https://doi.org/10.3390/molecules171213712
APA StyleLuongo, L., Petrelli, R., Gatta, L., Giordano, C., Guida, F., Vita, P., Franchetti, P., Grifantini, M., Novellis, V. D., Cappellacci, L., & Maione, S. (2012). 5'-Chloro-5'-deoxy-(±)-ENBA, a Potent and Selective Adenosine A1 Receptor Agonist, Alleviates Neuropathic Pain in Mice Through Functional Glial and Microglial Changes without Affecting Motor or Cardiovascular Functions. Molecules, 17(12), 13712-13726. https://doi.org/10.3390/molecules171213712