An Electrostatically Crosslinked Chitosan Hydrogel as a Drug Carrier
Abstract
:1. Introduction
2. Results and Discussion
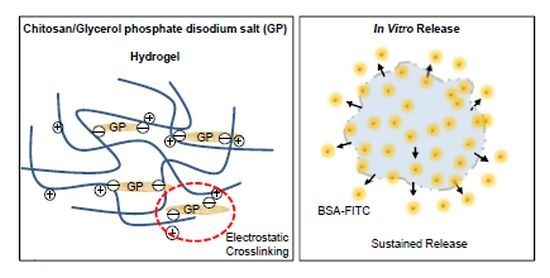
2.1. Formation of Chitosan/GP Hydrogel
2.2. In Vitro BSA Release
3. Experimental
3.1. Preparation of Chitosan Solution with and without GP
3.2. Characterization of the Interaction between Chitosan and GP Solution
3.3. Determination of the Sol-to-Gel Transition
3.4. Viscosity Measurements
3.5. In Vitro BSA-FITC Release
4. Conclusions
References
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-pass metabolism via UDP-glucuronosyltransferase: A barrier to oral bioavailability of phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Li, X. Stimuli-triggered structural engineering of synthetic and biological polymeric assemblies. Prog. Polym. Sci. 2012, 37, 1130–1176. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Bajpai, J.; Saini, R.; Gupta, R. Responsive polymers in biology and technology. Polym. Rev. 2011, 51, 53–97. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.J.; Chun, H.J.; Kim, C.H. Thermosensitive hydrogels for tissue engineering. Tissu. Eng. Reg. Med. 2011, 8, 117–123. [Google Scholar]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Zheng, L.; Luo, F.; Luo, J.; Qian, Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Zhang, Z.; Nan, K.; Li, L.; Wang, X.; Chen, H. Cytotoxicity and biocompatibility evaluation of N,O-carboxymethyl chitosan/oxidized alginate hydrogel for drug delivery application. Int. J. Biol. Macromol. 2012, 50, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev. 2011, 51, 1–30. [Google Scholar] [CrossRef]
- Santhagunam, A.; Madeira, C.; Cabral, J.M.S. Genetically engineered stem cell-based strategies for articular cartilage regeneration. Biotechnol. Appl. Biochem. 2012, 59, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, Z.; Wang, X.; Cheng, Q.; Wang, W.; Huang, X.; Zhou, J.; Zhang, Q.; Hou, L.; Huo, W. A biomimetic chitosan derivates: Preparation, characterization and transdermal enhancement studies of N-arginine chitosan. Molecules 2011, 16, 6778–6790. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.J.; Faneca, H.; Lima, M.P.; Gil, M.H.; Figueiredo, M.M. In situ forming chitosan hydrogels prepared via ionic/covalent co-cross-linking. Biomacromolecules 2011, 12, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| Solutions | Zeta Potential (mV) | Particle Size (nm) |
|---|---|---|
| GP only solution | −49.9 ± 17.1 | ND |
| Chitosan only solution (CGP-0) | 51.3 ± 36.7 | 400 ± 36 |
| CGP-10 | 13.6 ± 2.0 | 427 ± 3 |
| CGP-20 | 5.3 ± 2.0 | 597 ± 51 |
| CGP-30 | 3.6 ± 0.3 | 1070 ± 22 |
© 2012 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, G.O.; Kim, N.; Kim, D.Y.; Kwon, J.S.; Min, B.-H. An Electrostatically Crosslinked Chitosan Hydrogel as a Drug Carrier. Molecules 2012, 17, 13704-13711. https://doi.org/10.3390/molecules171213704
Kim GO, Kim N, Kim DY, Kwon JS, Min B-H. An Electrostatically Crosslinked Chitosan Hydrogel as a Drug Carrier. Molecules. 2012; 17(12):13704-13711. https://doi.org/10.3390/molecules171213704
Chicago/Turabian StyleKim, Ga On, Nawoo Kim, Da Yeon Kim, Jin Seon Kwon, and Byoung-Hyun Min. 2012. "An Electrostatically Crosslinked Chitosan Hydrogel as a Drug Carrier" Molecules 17, no. 12: 13704-13711. https://doi.org/10.3390/molecules171213704
APA StyleKim, G. O., Kim, N., Kim, D. Y., Kwon, J. S., & Min, B. -H. (2012). An Electrostatically Crosslinked Chitosan Hydrogel as a Drug Carrier. Molecules, 17(12), 13704-13711. https://doi.org/10.3390/molecules171213704