Tandem Three-Component Reactions of Aldehyde, Alkyl Acrylate, and Dialkylmalonate Catalyzed by Ethyl Diphenylphosphine
Abstract
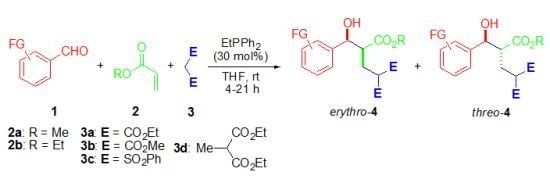
:1. Introduction

2. Results and Discussion

| Entry | CH2(CO2Et)2 (3a) (equiv.) | Catalyst | Solvent | Time (h) | dr of 4a b | Yield of 4a (%) c |
|---|---|---|---|---|---|---|
| 1 | 1.2 | EtPPh2 | t-BuOH | 0.5 | 21:79 | 47 |
| 2 | 1.2 | PPh3 | t-BuOH | 24 | - | Trace |
| 3 | 1.2 | PBu3 | t-BuOH | 4 | - | Trace d |
| 4 | 1.2 | DABCO | t-BuOH | 24 | 33:67 | 25 |
| 5 | 1.2 | EtPPh2 | i-PrOH | 2.5 | 25:75 | 38 |
| 6 | 1.2 | EtPPh2 | CH2Cl2 | 3 | 12:88 | 38 |
| 7 | 1.2 | EtPPh2 | Toluene | 4 | 15:85 | 51 |
| 8 | 1.2 | EtPPh2 | THF | 4 | 18:82 | 60 |
| 9 | 1.2 | EtPPh2e | THF | 2 | 12:88 | 49 |
| 10 | 1.2 | EtPPh2f | THF | 24 | 21:79 | 62 |
| 11 | 1.5 | EtPPh2 | THF | 5 | 16:84 | 54 |
| 12 g | 1.2 | EtPPh2 | THF/t-BuOH h | 1.5 | 19:81 | 43 |
| 13 | 1.2 | DMAP | THF | 24 | - | Trace |
| 14 | 1.2 | DBU | THF | 24 | - | Trace |

| Entry | Ar | R1 | R2 | Time (h) | dr of 4 b | Yield of 4 (%) c |
|---|---|---|---|---|---|---|
| 1 | 4-NO2C6H4 (1a) | CH3 (2a) | C2H5 (3a) | 4 | 18:82 | 4a (60) |
| 2 | 3-NO2C6H4 (1b) | 2a | 3a | 4 | 13:87 | 4b (63) |
| 3 | 2-NO2C6H4 (1c) | 2a | 3a | 4 | 37:63 | 4c (36) |
| 4 | 4-CF3C6H4 (1d) | 2a | 3a | 10 | 20:80 | 4d (57) |
| 5 | 4-CNC6H4 (1e) | 2a | 3a | 4 | 17:83 | 4e (50) |
| 6 | 3-Pyridyl (1f) | 2a | 3a | 21 | 18:82 | 4f (55) |
| 7 | 4-NO2C6H4 (1a) | C2H5 (2b) | 3a | 4 | 9:91 | 4g (50) |
| 8 | 3-NO2C6H4 (1b) | 2b | 3a | 4 | 15:85 | 4h (53) |
| 9 | 4-CNC6H4 (1e) | 2b | 3a | 6 | 11:89 | 4i (51) |
| 10 | 3-Pyridyl (1f) | 2b | 3a | 20 | 14:86 | 4j (52) |
| 11 | 4-NO2C6H4 (1a) | CH3 (2a) | CH3 (3b) | 5 | 13:87 | 4k (39) |


3. Experimental
3.1. General
3.2. Typical Synthetic Procedure
3.2.1. Optimization of Reaction Conditions for an Organocatalytic Three-Component Reaction of 4-Nitrobenzaldehyde (1a), Methyl Acrylate (2a), and Diethyl Malonate (3a) (TP for Table 1)
3.2.2. Typical Procedure for a Three-Component Reaction of Aromatic Aldehyde, Alkyl Acrylate, and Diethyl Malonate Catalyzed by EtPPh2 (TP for Table 2)
3.2.3. Procedure for Preparation of 5 (TP for Scheme 2)
3.2.4. Procedure for Preparation of 6 (TP for Scheme 3)
4. Conclusions
Supplementary Materials
Acknowledgements
- Sample Availability: Samples of all compounds are available from the authors.
References and Notes
- Dalko, P.I.; Moisan, L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar]
- Dalko, P.I.; Moisan, L. Enantioselective organocatalysis. Angew. Chem. Int. Ed. 2001, 40, 3726–3748. [Google Scholar]
- Zhu, J.; Bienaymé, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005.
- Dömling, A.; Ugi, I. Multicomponent reactions with isocyanides. Angew. Chem. 2000, 39, 3168–3210. [Google Scholar]
- Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Maximizing synthetic efficiency: Multi‐component transformations lead the way. Chem. Eur. J. 2000, 6, 3321–3329. [Google Scholar]
- Orru, R.V.A.; de Greef, M. Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 2003, 10, 1471–1799. [Google Scholar]
- Hulme, C.; Gore, V. Multi-component Reactions: Emerging chemistry in drug discover ‘From Xylocain to Crixivan’. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar]
- Ramón, D.J.; Yus, M. Asymmetric multicomponent reactions (AMCRs): The new frontier. Angew. Chem. Int. Ed. 2005, 44, 1602–1634. [Google Scholar]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar]
- Tietze, L.F.; Brasche, G.; Gericke, K. (Eds.) Domino Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006.
- Posner, G.H. Multicomponent one-pot annulations forming 3 to 6 bonds. Chem. Rev. 1986, 86, 831–844. [Google Scholar]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar]
- Basavaiah, D.; Reddy, B.S.; Badsara, S.S. Recent contributions from the Baylis-Hillman reaction to organic chemistry. Chem. Rev. 2010, 110, 5447–5674. [Google Scholar]
- Basavaiah, D.; Rao, K.V.; Reddy, R. The Baylis-Hillman reaction: A novel source of attraction, opportunities, and challenges in synthetic chemistr. Chem. Soc. Rev. 2007, 36, 1581–1588. [Google Scholar]
- Davie, E.A.C.; Mennen, S.M.; Xu, Y.; Miller, S.J. Asymmetric catalysis mediated by synthetic peptides. Chem. Rev. 2007, 107, 5759–5812. [Google Scholar]
- Masson, G.; Housseman, C.; Zhu, J. The enantioselective Morita-Baylis-Hillman reaction and its aza counterpart. Angew. Chem. Int. Ed. 2007, 46, 4614–4628. [Google Scholar]
- Basavaiah, D.; Rao, J.A.; Satyanarayana, T. Recent advances in the Baylis-Hillman reaction and applications. Chem. Rev. 2003, 103, 811–891. [Google Scholar]
- Langer, P. New strategies for the development of an asymmetric version of the Baylis-Hillman reaction. Angew. Chem. Int. Ed. 2000, 39, 3049–3052. [Google Scholar]
- Ciganek, E. The catalyzed α-hydroxyalkylation and α-aminoalkylation of activated olefins(The Morita-Baylis-Hillman Reaction). In Organic Reactions; Paquette, L.A., Ed.; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Singh, V.; Batra, S. Advances in the Baylis-Hillman reaction-assisted synthesis of cyclic frameworks. Tetrahedron 2008, 64, 4511–4574. [Google Scholar]
- Wang, W.; Yu, M. One-pot sequential Baylis-Hillman and Michael reactions. Tetrahedron Lett. 2004, 45, 7141–7143. [Google Scholar]
- Kim, S.C.; Lee, K.Y.; Lee, H.S.; Kim, J.N. Synthesis of poly-substituted benzenes starting from Baylis-Hillman adducts: DBU-assisted unusual dehydrogenation. Tetrahedron 2008, 64, 103–109. [Google Scholar]
- Roy, A.K.; Pathak, R.; Yadav, G.P.; Maulik, P.R.; Batra, S. Neighboring-group effect: DBU-promoted ring transformation of substituted isoxazoles to substituted pyrroles. Synthesis 2006, 1021–1027. [Google Scholar]
- Nag, S.; Yadav, G.P.; Maulik, P.R.; Batra, S. Sodium hydride mediated cascade reaction towards the synthesis of 1,5-disubstituted uracil from cyanamides derived from Baylis-Hillman adducts. Synthesis 2007, 911–917. [Google Scholar]
- Wang, D.-W.; Syu, S.; Hung, Y.-T.; Chen, P.; Lee, C.-J.; Chen, K.-W.; Chen, Y.-J.; Lin, W. Organocatalytic tandem three-component reaction of aldehyde, alkyl vinyl ketone, and amide: One-pot syntheses of highly functional alkenes. Org. Biomol. Chem. 2011, 9, 363–366. [Google Scholar]
- Syu, S.; Wang, D.-W.; Chen, P.; Hung, Y.-T.; Jhang, Y.-W.; Kao, T.-T.; Lee, Y.-T.; Lin, W. Tandem three-component reaction of aldehyde, alkyl acrylate, and amide using ethyl diphenylphosphine as organocatalyst. Tetrahedron Lett. 2010, 51, 5943–5946. [Google Scholar]
- Syu, S.; Lee, Y.-T.; Jang, Y.-J.; Lin, W. Organocatalytic tandem three-component reaction of imine, alkyl vinyl ketone, and imide via aza-Baylis-Hillman reaction. J. Org. Chem. 2011, 76, 2888–2891. [Google Scholar]
- The proposed mechanism concerning the phosphine catalysis process was not mentioned in this report. For detailed information, please see the references [25],[26],[27].
- Methot, J.L.; Roush, W.R. Nucleophilic phosphine organocatalysis. Adv. Synth. Cat. 2004, 346, 1035–1050. [Google Scholar]
- Wang, L.-C.; Luis, A.L.; Agapiou, K.; Jang, H.-Y.; Krische, M.J. Organocatalytic michael cycloisomerization of bis (enones): The intramolecular Rauhut-Currier reaction. J. Am. Chem. Soc. 2002, 124, 2402–2403. [Google Scholar]
- Lu, C.; Lu, X. Tandem reactions to construct heterocycles via phosphine-catalyzed umpolung addition and intramolecular conjugate addition. Org. Lett. 2002, 4, 4677–4679. [Google Scholar]
- Xu, L.-W.; Xia, C.-G. Highly efficient phosphine-catalyzed aza-Michael reactions of α,β-unsaturated compounds with carbamates in the presence of TMSCl. Tetrahedron Lett. 2004, 45, 4507–4510. [Google Scholar]
- Carolina, G.; Maria, L.; Caroline, M.; Marcial, M.-M.; Rosa, M.S.; Adelina, V. Michael additions catalyzed by phosphines. An overlooked synthetic method. Tetrahedron 2005, 61, 8598–8605. [Google Scholar]
- Yuan, K.; Song, H.-L.; Hu, Y.; Wu, X.-Y. Chiral phosphinothiourea-catalyzed asymmetric Morita-Baylis-Hillman reactions of acrylates with aromatic aldehydes. Tetrahedron 2009, 65, 8185–8190. [Google Scholar]
- The relative configuration of 5 was confirmed by X-ray analysis (CCDC number: 837000 for erythro-5). The stereochemistry of 4 and 6 was determined by 1H NMR analysis in comparison to 5.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jang, Y.-J.; Syu, S.-E.; Jhang, Y.-W.; Lee, Y.-T.; Lee, C.-J.; Chen, K.-W.; Das, U.; Lin, W. Tandem Three-Component Reactions of Aldehyde, Alkyl Acrylate, and Dialkylmalonate Catalyzed by Ethyl Diphenylphosphine. Molecules 2012, 17, 2529-2541. https://doi.org/10.3390/molecules17032529
Jang Y-J, Syu S-E, Jhang Y-W, Lee Y-T, Lee C-J, Chen K-W, Das U, Lin W. Tandem Three-Component Reactions of Aldehyde, Alkyl Acrylate, and Dialkylmalonate Catalyzed by Ethyl Diphenylphosphine. Molecules. 2012; 17(3):2529-2541. https://doi.org/10.3390/molecules17032529
Chicago/Turabian StyleJang, Yeong-Jiunn, Siang-En Syu, Yi-Wun Jhang, Yu-Ting Lee, Chia-Jui Lee, Ko-Wei Chen, Utpal Das, and Wenwei Lin. 2012. "Tandem Three-Component Reactions of Aldehyde, Alkyl Acrylate, and Dialkylmalonate Catalyzed by Ethyl Diphenylphosphine" Molecules 17, no. 3: 2529-2541. https://doi.org/10.3390/molecules17032529
APA StyleJang, Y. -J., Syu, S. -E., Jhang, Y. -W., Lee, Y. -T., Lee, C. -J., Chen, K. -W., Das, U., & Lin, W. (2012). Tandem Three-Component Reactions of Aldehyde, Alkyl Acrylate, and Dialkylmalonate Catalyzed by Ethyl Diphenylphosphine. Molecules, 17(3), 2529-2541. https://doi.org/10.3390/molecules17032529