Design, Synthesis and Antiproliferative Activity of Novel 2-Substituted-4-amino-6-halogenquinolines
Abstract
:1. Introduction

2. Results and Discussion
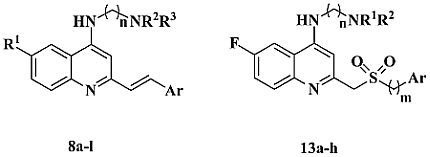
2.1. Chemistry

2.2. Biological Results and Discussion
| Compd. | R1 | n | NR2R3 | Ar | IC50 (μmol/L) | |||
|---|---|---|---|---|---|---|---|---|
| H-460 | HT-29 | HepG2 | SGC-7901 | |||||
| 8a | Cl | 3 |  |  | 1.57 | 2.04 | 2.89 | 3.34 |
| 8b | Cl | 3 |  |  | 2.10 | 2.36 | 1.76 | 4.21 |
| 8c | Cl | 2 |  |  | 0.46 | 0.79 | 0.93 | 1.56 |
| 8d | Cl | 3 |  |  | 1.58 | 2.35 | 3.03 | 3.30 |
| 8e | Cl | 2 |  |  | 0.03 | 0.55 | 0.33 | 1.24 |
| 8f | Cl | 3 |  |  | 1.20 | 1.74 | 1.86 | 1.55 |
| 8g | F | 2 |  |  | 0.51 | 1.03 | 0.66 | 1.33 |
| 8h | F | 3 |  |  | 0.49 | 0.96 | 0.77 | 1.98 |
| 8i | F | 2 |  |  | 0.91 | 1.84 | 1.56 | 1.72 |
| 8j | F | 3 |  |  | 1.97 | 1.68 | 4.88 | 5.60 |
| 8k | F | 2 |  |  | 3.82 | 5.27 | 4.51 | 11.84 |
| 8l | F | 3 |  |  | 2.32 | 2.46 | 1.78 | 4.74 |
| 1 | 3.52 | 1.35 | 2.06 | 4.92 | ||||
| gefitinib | 5.59 | 3.36 | 6.42 | 10.26 | ||||
| Compd. | m | n | NR1R2 | Ar | IC50 (μmol/L) | Compd. | m | n | NR1R2 | Ar | IC50 (μmol/L) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-460 | HT-29 | H-460 | HT-29 | ||||||||||
| 13a | 0 | 2 |  |  | 47.3 | 69.7 | 13f | 1 | 2 |  |  | 22.1 | 14.3 |
| 13b | 0 | 3 |  |  | NA a | 185.2 | 13g | 1 | 3 |  |  | 9.0 | 4.7 |
| 13c | 0 | 3 |  |  | 160.3 | NA a | 13h | 1 | 3 |  |  | 156.3 | NA a |
| 13d | 1 | 2 |  |  | 21.97 | 53.6 | 1 | 3.52 | 1.35 | ||||
| 13e | 1 | 3 |  |  | 146.9 | NA a | gefitinib | 5.59 | 3.36 | ||||
3. Experimental
3.1. Chemistry
3.2. General Procedure for the Preparation of Ethyl-(3Z)-3-(4-substitutedphenylamino)but-2-enoates 4a–b
3.3. General Procedure for the Preparation of 6-Substituted-2-methylquinolin-4-ols 5a–b
3.4. General Procedure for the Preparation of 6-Substituted-4-chloro-2-methylquinolines 6a–b
3.5. General Procedure for the Preparation of 6-Substituted-2-methyl-4-aminoquinolines 7a–f
3.6. General Procedure for the Preparation of Compounds 8a–l
3.7. 4-Chloro-6-fluoro-2-methylquinoline 1-oxide (9)
3.8. 4-Chloro-2-(chloromethyl)-6-fluoroquinoline (10)
3.9. General Procedure for the Preparation of Compounds 11a–d
3.10. General Procedure for the Preparation of Compounds 12a–d
3.11. General Procedure for the Preparation of Compounds 13a–h
3.12. Evaluation of the Biological Activity
4. Conclusions
Acknowledgments
- Samples Availability: Samples of the compounds are available from the authors.
References and Notes
- Abouzid, K.; Shouman, S. Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg. Med. Chem. 2008, 16, 7543–7551. [Google Scholar] [CrossRef]
- Fan, C.D.; Wang, W.W.; Zhao, B.X.; Zhang, S.L.; Miao, J.Y. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg. Med. Chem. 2006, 14, 3218–3222. [Google Scholar] [CrossRef]
- Sasaki, K.; Tsuno, N.H.; Sunami, E.; Tsurita, G.; Kawai, K.; Okaji, Y.; Nishikawa, T.; Shuno, Y.; Hongo, K.; Hiyoshi, M.; et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer 2010, 10, 370–380. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.L.; Deng, X.; Yang, S.; Mao, Y.; Li, Z.; Jiang, P.; Zhao, X.; Wei, Y. Chloroquine inhibits colon cancer cell growth in vitro and tumor growth in vivo via induction of apoptosis. Cancer Invest. 2009, 27, 286–292. [Google Scholar] [CrossRef]
- Munshi, A. Chloroquine in glioblastoma—New horizons for an old drug. Cancer 2009, 115, 2380–2383. [Google Scholar] [CrossRef]
- Strekowski, L.; Mokrosz, J.L.; Honkan, V.A.; Czarny, A.; Cegla, M.T.; Wydra, R.L.; Patterson, S.E.; Schinazi, R.F. Synthesis and quantitative structure-activity relationship analysis of 2-(aryl or heteroaryl)quinolin-4-amines, a new class of anti-HIV-1 agents. J. Med. Chem. 1991, 34, 1739–1746. [Google Scholar] [CrossRef]
- Strekowski, L.; Zegrocka, O.; Henary, M.; Say, M.; Mokrosz, M.J.; Kotecka, B.M.; Manzel, L.; Macfarlane, D.E. Structure-activity relationship analysis of substituted 4-quinolinamines, antagonists of immunostimulatory CpG-oligodeoxynucleotides. Bioorg. Med. Chem. Lett. 1999, 9, 1819–1824. [Google Scholar] [CrossRef]
- An, Z.Y.; Yan, Y.Y.; Peng, D.; Ou, T.M.; Tan, J.H.; Huang, S.L.; An, L.K.; Gu, L.Q.; Huang, Z.S. Synthesis and evaluation of graveoline and graveolinine derivatives with potent anti-angiogenesis activities. Eur. J. Med. Chem. 2010, 45, 3895–3903. [Google Scholar]
- Mo, L.P.; Liu, S.F.; Li, W.Z. An efficient method for the enamination of 1,3-dicarbonyl compounds with ceric ammonium nitrate (CAN). J. Chin. Chem. Soc. 2007, 54, 879–884. [Google Scholar]
- Nandhakumar, R.; Suresh, T.; Calistus Jude, A.L.; Rajesh kannan, V.; Mohan, P.S. Synthesis, antimicrobial activities and cytogenetic studies of newer diazepino quinoline derivatives via VilsmeiereHaack reaction. Eur. J. Med. Chem. 2007, 42, 1128–1136. [Google Scholar] [CrossRef]
- Moyer, M.P.; Weber, F.H.; Gross, J.L. Structure-activity relationships of imidazo[4,5-f]quinoline partial structures and analogs. Discovery of pyrazolo[3,4-f]quinoline derivatives as potent immunostimulants. J. Med. Chem. 1992, 35, 4595–4601. [Google Scholar]
- Strekowski, L.; Say, M.; Henary, M.; Ruiz, P.; Manzel, L.; Macfarlane, D.E.; Bojarski, A.J. Synthesis and activity of substituted 2-phenylquinolin-4-amines, antagonists of immunostimulatory CpG-oligodeoxynucleotides. J. Med. Chem. 2003, 46, 1242–1249. [Google Scholar] [CrossRef]
- Okano, M.; Mito, J.; Maruyama, Y.; Masuda, H.; Niwa, T.; Nakagawa, S.; Nakamura, Y.; Matsuura, A. Discovery and structure-activity relationships of 4-aminoquinazoline derivatives, a novel class of opioid receptor like-1 (ORL1) antagonists. Bioorg. Med. Chem. 2009, 17, 119–132. [Google Scholar] [CrossRef]
- Murphy, S.T.; Taylor, W.C.; Vadasz, A. Discovery and structure-activity relationships of 4-aminoquinazoline derivatives, a novel class of opioid receptor like-1 (ORL1) antagonists. Bioorg. Med. Chem. 1982, 35, 1215–1225. [Google Scholar]
- Arai, S.; Ishikura, M.; Sato, K. Synthesis of new azonia-helicenes with a quaternary nitrogen at the inner helix skeleton. J. Heterocycl. Chem. 1995, 32, 1081–1083. [Google Scholar] [CrossRef]
- Brasse, M.; Campora, J.; Palma, P. Nickel 2-iminopyridine N-oxide (PymNox) complexes: Cationic counterparts of salicylaldiminate-based neutral ethylene polymerization catalysts. Organometallics 2008, 27, 4711–4723. [Google Scholar] [CrossRef]
- White, J.D.; Yager, K.M.; Stappenbeck, F. Synthesis and conformation of 2-[3-(1-hydroxy hexyl)phenoxy]methyllquinoline, a 6-lipoxygenase inhibitor and leukotriene antagonist. J. Org. Chem. 1993, 58, 3466–3468. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhao, Y.F.; Zhai, X.; Liu, X.P.; Sun, L.X.; Ren, Y.X.; Gong, P. Synthesis and anti-HBV activities evaluation of new ethyl 8-imidazolylmethyl-7-hydroxyquinoline-3-carboxylate derivatives in vitro. Arch. Pharm. Chem. Life Sci. 2008, 341, 446–452. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, N.; Zhai, X.; Li, T.; Liu, D.; Zhang, T.; Wang, B.; Gong, P. Design, Synthesis and Antiproliferative Activity of Novel 2-Substituted-4-amino-6-halogenquinolines. Molecules 2012, 17, 5870-5881. https://doi.org/10.3390/molecules17055870
Jiang N, Zhai X, Li T, Liu D, Zhang T, Wang B, Gong P. Design, Synthesis and Antiproliferative Activity of Novel 2-Substituted-4-amino-6-halogenquinolines. Molecules. 2012; 17(5):5870-5881. https://doi.org/10.3390/molecules17055870
Chicago/Turabian StyleJiang, Nan, Xin Zhai, Ting Li, Difa Liu, Tingting Zhang, Bin Wang, and Ping Gong. 2012. "Design, Synthesis and Antiproliferative Activity of Novel 2-Substituted-4-amino-6-halogenquinolines" Molecules 17, no. 5: 5870-5881. https://doi.org/10.3390/molecules17055870
APA StyleJiang, N., Zhai, X., Li, T., Liu, D., Zhang, T., Wang, B., & Gong, P. (2012). Design, Synthesis and Antiproliferative Activity of Novel 2-Substituted-4-amino-6-halogenquinolines. Molecules, 17(5), 5870-5881. https://doi.org/10.3390/molecules17055870