Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics
Abstract
:1. Introduction

2. Results and Discussion
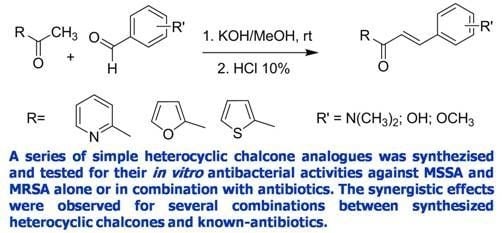
2.1. Chemistry

2.2. Antimicrobial Activity
| Compounds | A ring (R) | Substitution in B ring | Formula | Isolated yield (%) | |||
|---|---|---|---|---|---|---|---|
| C2 | C3 | C4 | C5 | ||||
| p1 |  | H | NO2 | H | H | C14H10N2O3 | 45 |
| p2 | H | H | N(CH3)2 | H | C16H16N2O | 62 | |
| p3 | H | H | OCH3 | OCH3 | C16H15NO3 | 58 | |
| p4 | H | OCH3 | OCH3 | OCH3 | C17H17NO4 | 51 | |
| p5 | OH | H | H | H | C14H11NO2 | 51 | |
| p6 | H | OH | H | H | C14H11NO2 | 50 | |
| p7 | H | H | OH | H | C14H11NO2 | 58 | |
| f1 |  | H | NO2 | H | H | C13H9NO4 | 43 |
| f2 | H | H | N(CH3)2 | H | C15H15O2 | 61 | |
| f3 | H | H | OCH3 | OCH3 | C15H14O4 | 55 | |
| f4 | H | OCH3 | OCH3 | OCH3 | C16H16O3 | 51 | |
| f5 | OH | H | H | H | C13H10O5 | 54 | |
| f6 | H | OH | H | H | C13H10O3 | 58 | |
| f7 | H | H | OH | H | C13H10O3 | 62 | |
| t1 |  | H | NO2 | H | H | C13H9NO3S | 46 |
| t2 | H | H | N(CH3)2 | H | C15H15NOS | 63 | |
| t3 | H | H | OCH3 | OCH3 | C15H14O3S | 50 | |
| t4 | H | OCH3 | OCH3 | OCH3 | C16H16O4S | 53 | |
| t5 | OH | H | H | H | C13H10O2S | 55 | |
| t6 | H | OH | H | H | C13H10O2S | 52 | |
| t7 | H | H | OH | H | C13H10O2S | 58 | |
| Chalcones a\Bacteria | Pyridin-2yl-chalcones | Furan-2yl-chalcones | Thiophen-2yl-chalcones | |||||
|---|---|---|---|---|---|---|---|---|
| p5 | p6 | p6 | f5 | f6 | f7 | t5 | t6 | |
| MSSA ATCC 25923 | 128 | 64 | 512 | 64 | 128 | 256 | 32 | 256 |
| MRSA ATCC 44330 | 128 | 128 | 512 | 64 | 128 | 256 | 64 | 128 |
| MRSA-I (isolated) b | - | 64 | - | 32 | 512 | - | 256 | - |
| Antibiotics | p6 | f5 | f6 | t5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | MRSA | Isolated MRSA | SA | MRSA | Isolated MRSA | SA | MRSA | Isolated MRSA | SA | MRSA | Isolated MRSA | |
| Vancomycin | - | + | - | - | + | - | - | + | - | + | + | - |
| Doxycycline | - | - | - | - | - | - | - | - | - | - | - | - |
| Ciprofloxacin | + | - | - | - | - | - | + | - | - | - | - | - |
| Gentamicin | - | - | - | - | - | - | - | - | - | - | - | - |
| Chloramphenicol | - | - | - | - | - | - | - | - | - | - | - | - |
| Oxacicllin | - | - | - | - | + | - | - | + | - | - | + | - |
| Erythromycin | - | - | - | - | - | - | - | - | - | + | - | - |
| Mix a | MSSA ATCC 25923 | MRSA ATCC 43300 | ||||||||
| MICs (µg/mL) | FICI | Interpretation | Increasing rate (fold) b | MICs (µg/mL) | FICI | Interpretation | Increasing rate (fold) b | |||
| Alone | Mix a | Alone | Mix a | |||||||
| p6 | 64 | 32 | 0.75 | Additive | ND | ND | ND | ND | ND | |
| Cipro | 0.5 | 0.125 | ||||||||
| p6 | ND | ND | ND | ND | ND | 128 | 32 | 0.31 | Synergistic | 4 |
| Vanco | 1 | 0.0625 | 16 | |||||||
| f5 | ND | ND | ND | ND | ND | 64 | 16 | 0.31 | Synergistic | 4 |
| Oxa | 2 | 0.125 | 16 | |||||||
| f6 | 128 | 64 | 0.75 | Additive | 2 | ND | ND | ND | ND | ND |
| Cipro | 0.5 | 0.125 | 4 | |||||||
| f6 | ND | ND | ND | ND | ND | 128 | 64 | 0.625 | Additive | 2 |
| Vanco | 1 | 0.125 | 8 | |||||||
| f6 | ND | ND | ND | ND | ND | 128 | 64 | 0.56 | Additive | 2 |
| Oxa | 2 | 0.125 | 16 | |||||||
| t5 | 32 | 32 | 1.06 | Indifferent | 1 | ND | ND | ND | ND | ND |
| Ery | 0.5 | 0.03 | 1.67 | |||||||
| t5 | 32 | 16 | 1 | Additive | 2 | ND | ND | ND | ND | ND |
| Cipro | 0.5 | 0.25 | 2 | |||||||
| t5 | ND | ND | ND | ND | ND | 64 | 32 | 1.0 | Additive | 2 |
| Vanco | 1 | 0.5 | 2 | |||||||
| t5 | ND | ND | ND | ND | ND | 64 | 32 | 0.56 | Additive | 2 |
| Oxa | 2 | 0.125 | 16 | |||||||
- (i) A free hydroxyl group in position 2/3 in the B ring (phenyl moiety) appears to be very important for anti-Staphylococcus aureus activity (p5, p6, f5, f6, t5). In case of substitution of a hydroxyl group in position 4 (p7, f7, t7), chalcones were inactive on the tested Staphylococcus aureus strains.
- (ii) Substitution in the B ring with a nitro group in position 3 (as in p1, f1, t1) or with two/three methoxyl groups in positions 3/4/5 (as in p3, p4, f3, f4, t3 and t4) might also be responsible for the decrease in the anti-Staphylococcus aureus activity;
- (iii) The chalcones possessing two/three methoxy groups in B ring were inactive, regardless of their A ring structures. This means that methoxy groups seem to abolish the hydrophilic property of the phenol hydroxy moiety which can affect penetration of antibiotics through bacterial cell walls.
- (iv) The chalcones possessing a hydroxyl group in B ring (p6, f6 and t5) demonstrated strongly positive interactions with antibiotic such as ciprofloxacin on methicillin-sensitive-Staphylococcus aureus. These chalcones increased significantly the activity of combined antibiotics like vancomycin and oxacillin up to sixteen-fold on methicillin -resistant-Staphylococcus aureus. These combinations could lead to develop new treatments for MRSA infectious diseases;
- (v) The analysis of structural influence of A ring on anti-Staphylococcus aureus activity suggested that a furan-2-yl moiety may be more important than the thiophene-2-yl or pyridine-2yl- moieties.
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Chalcones [14,30]
3.3. Antibacterial Activity
3.3.1. Antibiotics and Antibacterial Agents
3.3.2. Antibacterial Susceptibility Testing
3.2.3. Qualitative Determination of the Interaction between the Chalcones and Antibiotics
3.2.4. Measurement of MIC Values
3.2.5. Evaluation of Combined Activity

4. Conclusions
Acknowledgements
Conflict of Interest
- Sample Availability: Samples of the compounds p1-7, f1-7 and t1-7 are available from the authors.
References and notes
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073. [Google Scholar]
- Michel, M.; Gutmann, L. Methicillin-resistant Staphyllococus aureus and vancomycin-resistan enterococci: therapeut realities and possibilities. Lancet 1997, 349, 1901–1906. [Google Scholar] [CrossRef]
- Normark, B.H.; Normark, S. Evolution and spread of antibiotic resistance. J. Intern. Med. 2002, 252, 91–106. [Google Scholar] [CrossRef]
- Shekarchi, M.; Pirali-Hamedani, M.; Navidpour, L.; Adib, N.; Shafiee, A. Synthesis, Antibacterial and Antifungal Activities of 3-Aryl-5-(pyridin-3-yl)-4,5-dihydropyrazole-1-carbothioamide Derivatives. J. Iran. Chem. Soc. 2008, 5, 150–158. [Google Scholar] [CrossRef]
- Silverman, R.B. Organic Chemistry of Drug Design and Drug Action; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Thompson, L.A.; Ellman, J.A. Synthesis and applications of small molecule libraries. Chem. Rev. 1996, 96, 555–600. [Google Scholar]
- Alcaraz, L.E.; Blanco, S.E.; Puig, O.N.; Tomas, F.; Ferretti, F.H. Antibacterial activity of Flavonoids against methicillin- resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef]
- Baviskar, B.A.; Baviskar, B.; Shiradkar, M.R.; Deokate, U.A.; Khadabadi, S.S. Antimicrobial activity of some novel. Benzimidazolyl chalcones. E-J. Chem. 2009, 6, 196–200. [Google Scholar] [CrossRef]
- Echeverria, C.; Santibañez, J.F.; Donoso-Tauda, O.; Escobar, C.A.; Ramirez-Tagle, R. Structural antitumoral activity relationships of synthetic chalcones. Int. J. Mol. Sci. 2009, 10, 221–231. [Google Scholar]
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar] [CrossRef]
- Vogel, S.; Heilmann, J. Synthesis, cytotoxicity, and antioxidative activity of minor prenylated chalcones from Humulus lupulus. J. Nat. Prod. 2008, 71, 1237–1241. [Google Scholar] [CrossRef]
- Babasaheb, P.B.; Sachin, A.P.; Rajesh, N.G. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2010, 20, 730–733. [Google Scholar] [CrossRef]
- Vogel, S.; Barbic, M.; Jürgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef]
- Tran, T.-D.; Park, H.; Kim, H.P.; Ecker, G.F.; Thai, K.-M. Inhibitory activity of prostaglandin E2 production by the synthetic 2'-hydroxychalcone analogues: Synthesis and SAR study. Bioorg. Med. Chem. Lett. 2009, 19, 1650–1653. [Google Scholar] [CrossRef]
- Beom-Tae, K.; Kwang-Joong, O.; Jae-Chul, C.; Ki-Jun, H. Synthesis of dihydroxylated chalcone derivatives with diverse substitution patterns and their radical scavenging ability toward DPPH free radicals. Bull. Korean Chem. Soc. 2008, 29, 1125–1130. [Google Scholar] [CrossRef]
- Doan, T.N.; Tran, T.-D. Synthesis, antioxidant and antimicrobial activities of a novel series of chalcones, pyrazolic chalcones, and allylic chalcones. Pharmacol. Pharm. 2011, 2, 282–288. [Google Scholar]
- Sivakumar, P.M.; Prabhakar, P.K.; Doble, M. Synthesis, antioxidant evaluation, and quantitative structure-activity relationship studies of chalcones. Med. Chem. Res. 2011, 20, 482–492. [Google Scholar] [CrossRef]
- Vogel, S.; Ohmayer, S.; Brunner, G.; Heilmann, J. Natural and non-natural prenylated chalcones: synthesis, cytotoxicity and anti-oxidative activity. Bioorg. Med. Chem. 2008, 16, 4286–4293. [Google Scholar] [CrossRef]
- Munawar, M.A.; Azad, M.; Siddiqui, H.L. Synthesis and antimicrobial studies of some quinolinylpyrimidine derivatives. J. Chin. Soc. 2008, 55, 394–400. [Google Scholar]
- Azad, M.; Munawar, M.A.; Siddiqui, H.L. Antimicrobial activity and synthesis of quinoline-base chalcones. J. Appl. Sci. 2007, 7, 2485–2489. [Google Scholar] [CrossRef]
- Kalirajan, R.; Sivakumar, S.U.; Jubie, S.; Gowramma, B.; Suresh, B. Synthesis and biological evaluation of some heterocyclic derivatives of chalcones. Int. J. ChemTech Res. 2009, 1, 27–34. [Google Scholar]
- Talia, J.M.; Debattista, N.B.; Pappano, N.B. New antimicrobial combinations: Substituted chalcones- oxacillin against methicillin resistant Staphylococcus aureus. Braz. J Microbiol. 2011, 42, 470–475. [Google Scholar] [CrossRef]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J. Appl. Microbiol. 2012, 112, 55–64. [Google Scholar] [CrossRef]
- Sato, Y.; Shibata, H.; Arakaki, N.; Higuti, T. 6,7-dihydroxyflavone dramatically intensifies the susceptibility of methicillin-resistant or -sensitive Staphylococcus aureus to beta-lactams. Antimicrob. Agents Chemother. 2004, 48, 1357–1360. [Google Scholar] [CrossRef]
- Liu, M.H.; Otsuka, N.; Noyori, K.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Synergistic effect of kaempferol glycosides purified from Laurus nobilis and fluoroquinolones on methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2009, 32, 489–492. [Google Scholar] [CrossRef]
- Tiwari, B.; Pratapwar, A.S.; Tapas, A.R.; Butle, S.R.; Vatkar, B.S. Synthesis and antimicrobial activity of some chalcone derivatives. Int. J. ChemTech Res. 2010, 2, 499–503. [Google Scholar]
- Sivakumar, P.M.; Priya, S.; Doble, M. Synthesis, biological evaluation, mechanism of action and quantitative structure-activity relationship studies of chalcones as antibacterial agents. Chem. Biol. Drug Des. 2009, 73, 403–415. [Google Scholar] [CrossRef]
- Zheng, C.J.; Jiang, S.M.; Chen, Z.H.; Ye, B.J.; Piao, H.R. Synthesis and anti-bacterial activity of some heterocyclic chalcone derivatives bearing thiofuran, furan, and quinoline moieties. Arch. Pharm. Chem. Life Sci. 2011, 344, 689–695. [Google Scholar] [CrossRef]
- Lopez, S.N.; Castelli, M.V.; Zacchino, S.A.; Domnguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortes, J.C.G.; Ribas, J.C.; Devia, C.; Rodrguez, A.M.; et al. In Vitro Antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Funiss, B.S.; Hannford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed; Prentice Hall: Upper Saddle River: NJ, USA, 2004; pp. 1032–1035. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard M27-A2, 3rd ed; NCCLS: Wayne, PA, USA, 2002; Volume 22, pp. 1–30.
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 36, 493–496. [Google Scholar]
- Jorgensen, J.H.; Turnidge, J.D. Manual of Clinical Microbiology, 9th; Murray, P.R., Baron, E.J., Jorgensen, J.H., Landry, M.L., Pfaller, M.A., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 1152–1172. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved standard M7-A6, 6th ed; NCCLS: Wayne, PA, USA, 2003.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing. Informational supplement M100-S13, 12th ed; NCCLS: Wayne, PA, USA, 2003.
- Bajaksouzian, S.; Visalli, M.A.; Jacobs, M.R.; Appelbaum, P.C. Activities of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with amikacin, against acinetobacters as determined by checkerboard and time-kill studies. Antimicrob. Agents Chemother. 1997, 41, 1073–1076. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tran, T.-D.; Nguyen, T.-T.-N.; Do, T.-H.; Huynh, T.-N.-P.; Tran, C.-D.; Thai, K.-M. Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics. Molecules 2012, 17, 6684-6696. https://doi.org/10.3390/molecules17066684
Tran T-D, Nguyen T-T-N, Do T-H, Huynh T-N-P, Tran C-D, Thai K-M. Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics. Molecules. 2012; 17(6):6684-6696. https://doi.org/10.3390/molecules17066684
Chicago/Turabian StyleTran, Thanh-Dao, Thi-Thao-Nhu Nguyen, Tuong-Ha Do, Thi-Ngoc-Phuong Huynh, Cat-Dong Tran, and Khac-Minh Thai. 2012. "Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics" Molecules 17, no. 6: 6684-6696. https://doi.org/10.3390/molecules17066684
APA StyleTran, T.-D., Nguyen, T.-T.-N., Do, T.-H., Huynh, T.-N.-P., Tran, C.-D., & Thai, K.-M. (2012). Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics. Molecules, 17(6), 6684-6696. https://doi.org/10.3390/molecules17066684