Chemistry and Applications of Polysaccharide Solutions in Strong Electrolytes/Dipolar Aprotic Solvents: An Overview
Abstract
:1. Scope of the Overview
2. Introduction: Relevance to Green Chemistry
3. Derivatization of Cellulose, Chitin/Chitosan, and Starch
3.1. Relevance of the Molecular Structures of Cellulose, Chitin/Chitosan, and Starch to Biopolymer Processing and Derivatization
3.2. Principles of Polysaccharide Derivatization Under Heterogeneous and Homogeneous Reaction Conditions: Strong Electrolytes in Dipolar Aprotic Solvents
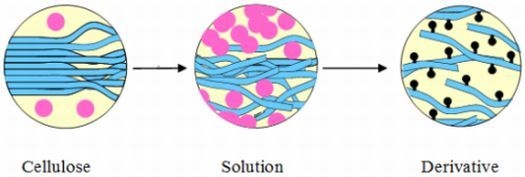
3.2.1. Derivatization of Cellulose Under Homogeneous Reaction Conditions
3.2.1.1. Strategies for Cellulose Activation: Solvent Exchange; Water Entrainment by Partial Solvent Distillation; Thermal Activation
Activation by Solvent Exchange
Water Entrainment by Partial Solvent Distillation
Thermal Activation
3.2.1.2. Mechanism of Cellulose Dissolution
3.2.1.3. Cellulose Derivatization
3.2.2. Dissolution and Derivatization of Chitin/Chitosan and Starch
4. Concluding Remarks
Acknowledgments
Abbreviations and Symbols
References
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of lignocellulosic biomass into furans for fuels and chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.; Whistler, R. Starch Chemistry and Technology, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2009; p. 879. [Google Scholar]
- Van Zyl, W.H.; Chimphango, A.F.A.; den Haan, R.; Goergens, J.F.; Chirwa, P.W.C. Next generation cellulosic ethanol technologies and their contribution to a sustainable Africa. Interface Focus 2011, 1, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, A.; Winkenbach, R.; Ehmsen, H. Material efficiency and sustainable development in chemistry: Where do we stand today? Chem. Ing. Tech. 2011, 83, 295–305. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Tharanathan, R.N. Chitin/chitosan Safe, ecofriendly packaging materials with multiple potential uses. Food Rev. Int. 2007, 23, 53–72. [Google Scholar] [CrossRef]
- Cao, X.; Sessa, J.D.; Wolf, J.W.; Willett, L.J. Static and dynamic solution properties of corn amylose in N,N-dimethylacetamide with 3% LiCl. Macromolecules 2000, 33, 3314–3323. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S. Reflections on the solubility of Cellulose. Ind. Eng. Chem. Res. 2010, 49, 11121–11130. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.-G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in) solubility: reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Malm, C.J.; Tanghe, L.O.; Laird, B.C.; Smith, G.D. Relative rates of acetylation of the hydroxyl groups in cellulose acetate. J. Am. Chem. Soc. 1953, 75, 80–84. [Google Scholar] [CrossRef]
- Nawaz, H.; Casarano, R.; El Seoud, O.A. First report on the kinetics of the uncatalyzed esterification of cellulose under homogeneous reaction conditions: a rationale for the effect of carboxylic acid anhydride chain-length on the degree of biopolymer substitution. Cellulose 2012, 19, 199–207. [Google Scholar] [CrossRef]
- Klemm, D.; Phillip, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry; Wiley-VCH: Weinheim, Germany, 1998; Volume 1, p. 133. [Google Scholar]
- Law, R.C. Cellulose acetate in textile application. Macromol. Symp. 2004, 208, 255–265. [Google Scholar] [CrossRef]
- De Vasconcelos, C.L.; Bezerril, P.M.; Pereira, M.R.; Ginani, M.F.; Fonseca, J.L.C. Viscosity-temperature behavior of chitin solutions using lithium chloride/DMA as solvent. Carbohydr. Res. 2011, 346, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Majeti, N.V.; Kumar, R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar]
- Noishiki, Y.; Takami, H.; Nishiyama, Y.; Wada, M.; Okada, S.; Kuga, S. Alkali induced conversion of beta-chitin to alpha-chitin. Biomacromolecules 2003, 4, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Toffey, A.; Samaranayake, G.; Frazier, C.E.; Glasser, W.G. Chitin derivatives. I. Kinetics of the heat induced conversion of chitosan to chitin. J. Appl. Polym. Sci. 1996, 60, 75–85. [Google Scholar] [CrossRef]
- Khor, E.; Wu, H.; Lim, L.Y.; Guo, C.M. Chitin-Methacrylate: Preparation, Characterization and Hydrogel Formation. Materials 2011, 4, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, P.C. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Liu, Q. Understanding Starches and Their Role in Foods. p. 316. Available online: http://uqu.edu.sa/files2/tiny_mce/plugins/filemanager/files/4300270/1/2/1574_C007.pdf (accessed on 20 December 2012).
- Brown, A.C. Understanding Food: Principles and Preparation. 2007, p. 362. Available online: http://books.google.com.br/books?id=edPzm5KSMmYC&pg=PA360&source=gbs_toc_r&cad=4#v=onepage&q&f=false (accessed on 20 December 2012).
- Mitchell, J. Starch as a hydrocolloid. Available online: http://www.stepitn.eu/wpcontent/uploads/2010/06/pm18_Unilever_Mitchell_1.pdf (accessed on 20 December 2012).
- Singha, J.; Kaurb, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Diamantoglou, M.; Vienken, J. Strategies for the development of hemocompatible dialysis membranes. Macromol. Symp. 1996, 103, 31–42. [Google Scholar] [CrossRef]
- Moellmann, E.; Heinze, T.; Liebert, T.; Sarah, K. Homogeneous synthesis of cellulose ethers in ionic liquids. US 20090221813 3 September 2009. [Google Scholar]
- Ramos, L.A.; Assaf, J.M.; El Seoud, O.A.; Frollini, E. Influence of the supra-molecular structure and physico-chemical properties of cellulose on its dissolution in the lithium chloride/N,N-dimethylacetamide solvent system. Biomacromolecules 2005, 6, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Heinze, T. Organic esters of cellulose: New perspectives for old polymers. Adv. Polym. Sci. 2005, 186, 103–149. [Google Scholar]
- Chaudemanche, C.; Navard, P. Swelling and dissolution mechanisms of regenerated Lyocell cellulose fibers. Cellulose 2011, 18, 1–15. [Google Scholar] [CrossRef]
- Cuissinat, C.; Navard, P. Swelling and Dissolution of Cellulose Part II: Free Floating Cotton and Wood Fibres in NaOH-Water-Additives Systems. Macromol. Symp. 2006, 244, 19–30. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of Ionic Liquids in Carbohydrate Chemistry: A Window of Opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Dicke, R.; Koschella, A.; Kull, A.H.; Klohr, E.-A.; Koch, W. Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol. Chem. Phys. 2000, 201, 627–631. [Google Scholar] [CrossRef]
- Köhler, S.; Heinze, T. New Solvents for Cellulose: Dimethyl Sulfoxide/Ammonium Fluorides. Macromol. Biosci. 2007, 7, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Marson, G.A.; El Seoud, O.A. A Novel, Effecient Procedure for Acylation of Cellulose Under Homogeneous Solution Conditions. J. Appl. Polym. Sci. 1999, 74, 1355–1360. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Marson, G.A.; Ciacco, G.T.; Frollini, E. An Efficient, One-Pot Acylation of Cellulose under Homogeneous Reaction Conditions. Macromol. Chem. Phys. 2000, 201, 882–889. [Google Scholar] [CrossRef]
- Huglin, M. Light Scattering from Polymer Solutions; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Kwolek, S.; Morgan, P.W.; Schaefgen, J.R.; Gulrich, L.W. Synthesis, anisotropic solutions, and fibers of poly(1,4-benzamide). Macromolecules 1977, 10, 1390–1396. [Google Scholar] [CrossRef]
- Austin, P.R. Chitin solutions. U.S. Patent No. 4059457, 22 December 1977. [Google Scholar]
- Gagnaire, D.; Saint-Germain, J.; Vincendon, M. NMR evidence of hydrogen bonds in cellulose solutions. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 261–275. [Google Scholar]
- McCormick, C.L.; Lichatowich, D.K. Homogeneous solution reactions of cellulose, chitin, and other polysaccharides to produce controlled-activity pesticide systems. J. Polym. Sci. Polym. Lett. Ed. 1979, 17, 479–484. [Google Scholar] [CrossRef]
- McCormick, C.L. Cellulose solutions. U.S. Patent No. 4278790 A, 14 July 1981. [Google Scholar]
- Turbak, A.F.; El-Kafrawy, A.; Snyder, F.W.; Auerbach, A.B. Solvent system for cellulose. US Patent No. 4302252 A, 24 November 1981. [Google Scholar]
- Kennedy, G.L., Jr. Biological effects of acetamide, formamide, and their monomethyl and dimethyl derivatives: Critical review. Toxicology 1986, 17, 129–182. [Google Scholar] [CrossRef] [PubMed]
- Willhite, C.C.; Katz, P.I. Dimethyl sulfoxide. J. Appl. Toxicol. 1984, 4, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Manno, M.; Rugge, M.; Cocheo, V. Double fatal inhalation of dichloromethane. Hum. Exp. Toxicol. 1992, 11, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Bogan, R.T.; Brewer, R.J. Cellulose esters, organic. In Encylopedia of Polymer Science and Engineering; Kroschwitz, J.I., Bickford, M., Klingsberg, A., Muldoon, J., Salvatore, A., Eds.; Wiley-Interscience: New York, NY, USA, 1985; pp. 158–181. [Google Scholar]
- Sharma, R.K.; Fry, J.L. Instability of anhydrous tetra-n-alkylammonium fluorides. J. Org. Chem. 1983, 48, 2112–2114. [Google Scholar] [CrossRef]
- Callais, P.A. Derivatzation and Characterization of Cellulose in Lithium Chloride and N,N-Dimethylacetamide Solutions. Ph.D. Thesis, University of Southern Mississippi, Hattiesburg, MS, USA, 1986. [Google Scholar]
- Krässig, H. Ullman’s Encyclopedia of Industrial Chemistry, Campbell FT, 5th ed.; Pfefferkorn, R., Rousaville, J.F., Eds.; VCH: Weinheim, Germany, 1986; Volume 5, p. 375. [Google Scholar]
- McCormick, C.L.; Callais, P.A.; Hutchinson, B.H., Jr. Solution studies of cellulose in lithium chloride and N,N-dimethylacetamide. Macromolecules 1985, 18, 2394–2401. [Google Scholar] [CrossRef]
- Pionteck, H.; Berger, W.; Morgenstern, B.; Fengel, D. Changes in cellulose structure during dissolution in LiCl: N,N-dimethylacetamide and in the alkaline iron tartarate system EWNN. Ι. Electron microscopic studies on changes in cellulose morphology. Cellulose 1996, 3, 127–139. [Google Scholar] [CrossRef]
- Dawsey, T.R.; McCormick, C.L. The lithium chloride/N,N-dimethylacetamide solvent for cellulose: A literature review. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1990, C30, 405–440. [Google Scholar] [CrossRef]
- Ekmanis, J.L.; Turbak, A.F. Lab Highlights 251; Waters Chromatography Division: Milford, MA, USA, 1986. [Google Scholar]
- Ekmanis, J.L. Gel permeation chromatographic analysis of cellulose. Am. Lab. News 1987, 19, 10–11. [Google Scholar]
- Striegel, A.M.; Timpa, J.D. Size exclusion chromatography of polysaccharides in dimethylacetamide-lithium chloride. ACS Symp. Ser. 1996, 635, 366–378. [Google Scholar]
- Silva, A.A.; Laver, M.L. Molecular weight characterization of wood pulp cellulose: dissolution and size exclusion chromatographic analysis. Tappi J. 1997, 80, 173–180. [Google Scholar]
- Timpa, J.D. Application of universal calibration in gel permeation chromatography for molecular weight determinations of plant cell wall polymers: Cotton fiber. J. Agric. Food. Chem. 1991, 39, 270–275. [Google Scholar] [CrossRef]
- Marson, G.A.; El Seoud, O.A. Cellulose Dissolution in Lithium Chloride/N,N-Dimethylacetamide Solvent System: Relevance of Kinetics of Decrystallization to Cellulose Derivatization Under Homogeneous Solution Conditions. J. Polym. Sci. A Polym. Chem. 1999, 37, 3738–3744. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Kosma, P. Trapping of Reactive Intermediates to Study Reaction Mechanisms in Cellulose Chemistry. Adv. Polym. Sci. 2006, 205, 153–197. [Google Scholar]
- Regiani, A.M.; Frollini, E.; Marson, G.A.; Arantes, G.M.; El Seoud, O.A. Some Aspects of Acylation of Cellulose Under Homogeneous Solution Conditions. J. Polym. Sci. A Polym. Chem. 1999, 37, 1357–1363. [Google Scholar] [CrossRef]
- Marson, G. Acylation of cellulose under homogeneous reaction conditions. M.Sc. Thesis, University of São Paulo, São Paulo, Brazil, 1999. [Google Scholar]
- Berger, W.; Keck, M.; Phillip, B.; Schleicher, H. Nature of interactions in the dissolution of cellulose in nonaqueous solvent systems. Lenzinger Ber. 1985, 59, 88–95. [Google Scholar]
- El-Kafrawy, A. Investigation of the Cellulose/LiCl/Dimethylacetamide and Cellulose/LiC1/N-Methyl-2-Pyrrolidinone Solutions by 13C-NMR Spectroscopy. J. Appl. Polym. Sci. 1982, 27, 2435–2443. [Google Scholar] [CrossRef]
- Herlinger, H.; Hengstberger, M. Behavior of cellulose in unconventional solvents. Lenzinger Ber. 1985, 59, 96–104. [Google Scholar]
- Vincendon, M. Proton NMR study of the chitin dissolution mechanism . Makromol. Chem. 1985, 186, 1787–1795. [Google Scholar]
- Striegel, A.M. Theory and applications of DMAC/LiCl in the analysis of Polysaccharides. Carbohydr. Polym. 1997, 34, 267–274. [Google Scholar] [CrossRef]
- Heinze, T. New ionic polymers by cellulose functionalization. Macromol. Chem. Phys. 1998, 199, 2341–2364. [Google Scholar] [CrossRef]
- Striegel, A.M. Advances in the understanding of the dissolution mechanism of cellulose in LiCl/DMAC. J. Chil. Chem. Soc. 2003, 48, 73–77. [Google Scholar] [CrossRef]
- Lindman, B.; Karlstroem, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Morgenstern, B.; Kammer, H.W.; Berger, W.; Skrabal, P. Lithium-7 NMR study on cellulose/lithium chloride/N,N-dimethylacetamide solutions. Acta Polym. 1992, 43, 356–357. [Google Scholar] [CrossRef]
- Spange, S.; Reuter, A.; Vilsmeier, E.; Heinze, T.; Keutel, D.; Linert, W. Determination of Empirical Polarity Parameters of the Cellulose Solvent N,N-Dimethylacetamide/LiCl by means of the Solvatochromic Technique. J. Polym. Sci. A Polym. Chem. 1998, 36, 1945–1955. [Google Scholar] [CrossRef]
- Fidale, L.C.; Heinze, T.; El Seoud, O.A. Perichromism: A powerful tool for probing the properties of cellulose and its derivatives. Carbohydr. Polym 2013, in press. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, S.; Matsumoto, Y.; Kuga, S. Cellulose gel and aerogel from LiCl/DMSO solution. Cellulose 2012, 19, 393–399. [Google Scholar] [CrossRef]
- Pliego, J.R.; Pilo’-Veloso, D. Effects of ion-pairing and hydration on the SNAr reaction of the F- with p-chlorobenzonitrile in aprotic solvents. Phys. Chem. Chem. Phys. 2008, 10, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Chrapava, S.; Touraud, D.; Rosenau, T.; Potthast, A.; Kunz, W. The investigation of the influence of water and temperature on the LiCl/DMAC/cellulose system. Phys. Chem. Chem. Phys. 2003, 5, 1842–1847. [Google Scholar] [CrossRef]
- Ostlund, A.; Lundberg, D.; Nordstierna, L.; Holmberg, K.; Nyden, M. Dissolution and Gelation of Cellulose in TBAF/DMSO Solutions: The Roles of Fluoride Ions and Water. Biomacromolecules 2009, 10, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.A.; Morgado, D.L.; El Seoud, O.A.; da Silva, V.C.; Frollini, E. Acetylation of cellulose in LiCl-N,N-dimethylacetamide: first report on the correlation between the reaction efficiency and the aggregation number of dissolved cellulose. Cellulose 2011, 18, 385–392. [Google Scholar] [CrossRef]
- Striegel, A.M.; Timpa, J.D. Molecular characterization of polysaccharides dissolved in Me2 NAc-LiCl by gel-permeation chromatography. Carbohydr. Res. 1995, 267, 271–290. [Google Scholar] [CrossRef]
- Sjoholm, E.; Gustafsson, K.; Pettersson, B.; Colmsjo, A. Characterization of the cellulosic residues from lithium chloride/N,N-dimethylacetamide dissolution of softwood kraft pulp. Carbohydr. Polym. 1997, 32, 57–63. [Google Scholar] [CrossRef]
- Roder, T.; Morgenstern, B.; Schelosky, N.; Glatter, O. Solutions of cellulose in N,N-dimethylacetamide/lithium chloride studied by light scattering methods. Polymer 2001, 42, 6765–6773. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tatsumi, D.; Tamai, N.; Takaki, T. Solution properties of celluloses from different biological origins in LiCl /DMAc. Cellulose 2001, 8, 275–282. [Google Scholar] [CrossRef]
- Marsano, E.; Conio, G.; Martino, R.; Turturro, A.; Bianchi, E. Fibers Based on Cellulose-Chitin Blends. J. Appl. Polym. Sci. 2002, 83, 1825–1831. [Google Scholar] [CrossRef]
- Schult, T.; Hjerde, T.; Optun, O.I.; Kleppe, P.J.; Moe, S. Characterization of cellulose by SEC-MALLS. Cellulose 2002, 9, 149–158. [Google Scholar] [CrossRef]
- Strlic, M.; Kolar, J. Size exclusion chromatography of cellulose in LiCl/N,N-dimethylacetamide. J. Biochem. Biophys. Methods. 2003, 56, 265–279. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T. Effect of Solvent Exchange on the Solid Structure and Dissolution Behavior of Cellulose. Biomacromolecules 2003, 4, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Tamai, N.; Aono, H.; Tatsumi, D.; Matsumoto, T. Differences in Rheological Properties of Solutions of Plant and Bacterial Cellulose in LiCl/N,N-Dimethylacetamide. J. Soc. Rheol. Jpn. 2003, 31, 119–130. [Google Scholar] [CrossRef]
- Dupont, A.-L. Cellulose in lithium chloride/N,N-dimethylacetamide, optimisation of a dissolution method using paper substrates and stability of the solutions. Polymer 2003, 44, 4117–4126. [Google Scholar] [CrossRef]
- Tamai, N.; Tatsumi, D.; Matsumoto, T. Rheological Properties and Molecular Structure of Tunicate Cellulose in LiCl/1,3-Dimethyl-2-imidazolidinone. Biomacromolecules 2004, 5, 422–32. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A.-L.; Harrison, G. Conformation and dn/dc determination of cellulose in N,N-dimethylacetamide containing lithium chloride. Carbohydr. Polym. 2004, 58, 233–243. [Google Scholar] [CrossRef]
- Aono, H.; Tatsumi, D.; Matsumoto, T. Scaling Analysis of Cotton Cellulose/LiCl.DMAc Solution Using Light Scattering and Rheological Measurements. J. Polym. Sci. B Polym. Phys. 2006, 44, 2155–2160. [Google Scholar] [CrossRef]
- Benoit, J.C.Z.; Newman, R.H.; Staiger, M.P. Phase transformations in microcrystalline cellulose due to partial dissolution. Cellulose 2007, 14, 311–320. [Google Scholar]
- Marsano, E.; Canetti, M.; Conio, G.; Corsini, P.; Freddi, G. Fibers Based on Cellulose-Silk Fibroin Blend. J. Appl. Polym. Sci. 2007, 104, 2187–2196. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T. Effect of solvent exchange on the supramolecular structure, the molecular mobility and the dissolution behavior of cellulose in LiCl/DMAc. Carbohydr. Res. 2008, 343, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Aulin, C.; Ahola, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Oesterberg, M.; Wagberg, L. Nanoscale Cellulose Films with Different Crystallinities and Mesostructures;Their Surface Properties and Interaction with Water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef] [PubMed]
- Semsarilar, M.; Perrier, S. Solubilization and Functionalization of Cellulose Assisted by Microwave Irradiation. Aust. J. Chem. 2009, 62, 223–226. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Staiger, M.P.; Tucker, N.; Newman, R.H. Aerocellulose Based on All-Cellulose Composites. J. Appl. Polym. Sci. 2010, 115, 216–221. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Sian, C.; Gea, S.; Nishino, T.; Peijs, T. All-cellulose nanocomposites by surface selective dissolution of bacterial cellulose. Cellulose 2009, 16, 435–444. [Google Scholar] [CrossRef]
- Wang, Z.; Yokoyama, T.; Chang, H.; Matsumoto, Y. Dissolution of Beech and Spruce Milled Woods in LiCl/DMSO. J. Agric. Food Chem. 2009, 57, 6167–6170. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Kim, J. Multiwalled carbon nanotubes-cellulose paper for a chemical vapor sensor. Sens. Actuators B Chem. 2010, 150, 308–313. [Google Scholar] [CrossRef]
- John, A.; Mahadeva, S.K.; Kim, J. The preparation, characterization and actuation behavior of polyaniline and cellulose blended electro-active paper. Smart Mater. Struct. 2010, 19, 1–6. [Google Scholar] [CrossRef]
- Moigne, L.N.; Spinu, M.; Heinze, T.; Navard, P. Restricted dissolution and derivatization capacities of cellulose fibres under uniaxial elongatioanl stress. Polymer 2010, 51, 447–453. [Google Scholar] [CrossRef]
- Abbott, A.; Bismarck, A. Self-reinforced cellulose nanocomposites. Cellulose 2010, 17, 779–791. [Google Scholar] [CrossRef]
- Heinze, T.; Koehler, S. Dimethyl Sulfoxide and Ammonium Fluorides Novel Cellulose Solvents. ACS Symp. Series. 2010, 1033, 103–118. [Google Scholar]
- Yamamoto, M.; Kuramae, R.; Yanagisawa, M.; Ishii, D.; Isogai, A. Light-Scattering Analysis of Native Wood Holocelluloses Totally Dissolved in LiCl-DMI Solutions: High Probability of Branched Structures in Inherent Cellulose. Biomacromolecules 2011, 12, 3982–3988. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.W.; Cai, Z.J. A novel long-life air working electro-active actuator based on cellulose and polyurethane blend. Adv. Mater. Res. 2011, 298, 40–44. [Google Scholar] [CrossRef]
- Russler, A.; Sakakibara, K.; Rosenau, T. Cellulose as matrix component of conducting films. Cellulose 2011, 18, 937–944. [Google Scholar] [CrossRef]
- Ramos, L.A.; Morgado, D.L.; Gessner, F.; Frollini, E.; El Seoud, O.A. A physical organic chemistry approach to dissolution of cellulose: effects of cellulose mercerization on its properties and on the kinetics of its decrystallization. ARKIVOC 2011, 7, 416–425. [Google Scholar]
- Yousefi, H.; Faezipour, M.; Nishino, T.; Shakeri, A.; Ebrahimi, G. All-cellulose composite and nanocomposite made from partially dissolved micro-and nanofibers of canola straw. Polym. J. 2011, 43, 559–564. [Google Scholar] [CrossRef]
- Li, J.; Martin-Sampedro, R.; Pedrazzi, C.; Gellerstedt, G. Fractionation and characterization of lignin-carbohydrate complexes (LCCs) from eucalyptus fibers. Holzforschung 2011, 65, 43–50. [Google Scholar] [CrossRef]
- Ma, C.; Xu, X.-L.; Ai, P.; Xie, S.-M.; Lv, Y.-C.; Shan, H.-Q.; Yuan, L.-M. Chiral Separation of D, L-Mandelic Acid Through Cellulose Membranes. Chirality 2011, 23, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Henniges, U.; Kostic, M.; Borgards, A.; Rosenau, T.; Potthast, A. Dissolution Behavior of Different Celluloses. Biomacromolecules 2011, 12, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Opdenbosch, V.D.; Maisch, P.; Fritz-Popovski, G.; Paris, O.; Zollfrank, C. Transparent cellulose sheets as synthesis matrices for inorganic functional Particles. Carbohydr. Polym. 2012, 87, 257–264. [Google Scholar] [CrossRef]
- Saint, G.J.; Vincendon, M. 1H, 13C and 7Li Nuclear Magnetic Resonance Study of the Lithium Chloride-N,N-Dimethylacetamide System. Org. Magn. Reson. 1983, 21, 371–375. [Google Scholar]
- Petrus, L.; Gray, D.G.; BeMiller, J.N. Homogeneous alkylation of cellulose in lithium chloride/dimethyl sulfoxide solvent with dimsyl sodium activation. A proposal for the mechanism of cellulose dissolution in lithium chloride/DMSO. Carbohydr. Res. 1995, 268, 319–323. [Google Scholar] [CrossRef]
- Striegel, A.M.; Timpa, J.D.; Piotrowiak, P.; Cole, R.B. Multiple neutral alkali halide attachments onto oligosaccharides in electrospray ionization mass spectrometry. Int. J. Mass Spectrom. Ion Processes. 1997, 162, 45–53. [Google Scholar] [CrossRef]
- He, J.; Liu, Z.; Li, H.-Y.; Wang, G.; Pu, J. Solubility of wood-cellulose in LiCl/DMAC solvent system. For. Stud. China 2007, 9, 217–220. [Google Scholar]
- Wei, Y.; Cheng, F. Effect of Solvent Exchange on the Structure and Rheological Properties of Cellulose in LiCl/DMAC. J. Appl. Polym. Sci. 2007, 106, 3624–3630. [Google Scholar] [CrossRef]
- Jerosch, H.; Lavedrine, B.; Cherton, J. Study of the stability of cellulose-holocellulose solutions in N,N-dimethylacetamide-lithium chloride by size exclusion chromatography. J. Chromatogr. A 2001, 927, 31–38. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Sixta, H.; Kosma, P. Degradation of cellulosic materials by heating in LiCl/DMAC. Tetrahedron Lett. 2002, 43, 7757–7759. [Google Scholar] [CrossRef]
- Roder, T.; Potthast, A.; Rosenau, T.; Kosmsa, P.; Baldinger, T.; Morgenstern, B.; Glatter, O. The effect of water on cellulose solutions in LiCl/DMAC. Macromol. Symp. 2002, 190, 151–159. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Sartori, J.; Sixta, H.; Kosma, P. Hydrolytic processes and condensation reactions in the cellulose solvent system N,N-dimethylacetamide/lithium chloride. Part 2: Degradation of cellulose. Polymer 2003, 44, 7–17. [Google Scholar] [CrossRef]
- Aono, H.; Tamai, N.; Tatsumi, D.; Matsumoto, T. Aggregate stucture and rheological properties of mercerized cellulose/LiCl.DMAc solution. Nihon Reoroji Gakkaishi 2004, 32, 169–177. [Google Scholar] [CrossRef]
- Aono, H.; Tatsumi, D.; Matsumoto, T. Characterization of Aggregate Structure in Mercerized Cellulose in LiCl/DMAC Solution Using Light Scattering and Rheological Measurements. Biomacromolecules 2006, 7, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Ishii, D.; Tatsumi, D.; Matsumoto, T.; Murata, K.; Hayashi, H.; Yoshitani, H. Investigation of the Structure of Cellulose in LiCl/DMAc Solution and Its Gelation Behavior by Small-Angle X-Ray Scattering Measurements. Macromol. Biosci. 2006, 6, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cheng, F. Synthesis and aggregates of cellulose-based hydrophobically associating polymer. Carbohydr. Polym. 2007, 68, 734–739. [Google Scholar] [CrossRef]
- Morgado, D.L.; Martins, V.C.A.; Plepis, A.G.; Frollini, E. Aggregation of Chains of Cellulose Acetates in LiCl/DMAc: Evaluation via Viscometry. Polimeros 2011, 21, 143–145. [Google Scholar]
- Sjoholm, E.; Gustafsson, K.; Eriksson, B.; Brown, W.; Colmsjo, A. Aggregation of cellulose in lithium chloride, N,N-dimethylacetamide. Carbohydr. Polym. 2000, 41, 153–161. [Google Scholar] [CrossRef]
- Izatt, R.M.; Rytting, J.H.; Hansen, L.D.; Christensen, J.J. Thermodynamics of Proton Dissociation in Dilute Aqueous Solution. V. An Entropy Titration Study of Adenosine, Pentoses, Hexoses, and Related Compounds. J. Am. Chem. Soc. 1966, 88, 2641–2645. [Google Scholar] [CrossRef] [PubMed]
- Sealey, J.E.; Samaranayake, G.; Todd, J.G.; Glasser, W.G. Novel cellulose derivatives. IV. Preparation and thermal analysis of waxy esters of cellulose. J. Polym. Sci. B Polym. Phys. 1996, 34, 1613–1620. [Google Scholar] [CrossRef]
- Vaca-Garcia, C.; Thiebaud, S.; Borredon, M.E.; Gozzelino, G. Cellulose esterification with fatty acids, including oleic acid, and acetic anhydride in lithium chloride/N,N-dimethylacetamide medium. JAOCS 1998, 75, 315–319. [Google Scholar] [CrossRef]
- Peydecastaing, P.; Vaca-Garcia, C.; Borredon, E. Consecutive reactions in an oleic acid and acetic anhydride reaction medium. Eur. J. Lipid Sci. Technol. 2009, 111, 723–729. [Google Scholar] [CrossRef]
- Heinze, T.; Sarbova, V.; Nagel, V.C.M. Simple synthesis of mixed cellulose acylate phosphonates applying n-propyl phosphonic acid anhydride. Cellulose 2012, 19, 523–531. [Google Scholar] [CrossRef]
- Siegmund, G.; Klemm, D. Cellulose—Cellulose sulfonates: preparation, properties, subsequent reactions. Polymer News 2002, 27, 84–90. [Google Scholar]
- Nawaz, H.; Pires, P.A.R.; El Seoud, O.A. Kinetics and mechanism of imidazole-catalyzed acylation of cellulose in LiCl/N,N-dimethylacetamide. Carbohydr. Polym. 2013, 92, 997–1005. [Google Scholar]
- Guo, Y.; Wang, X.; Li, D.; Du, H.; Wang, X.; Sun, R. Synthesis and characterization of hydrophobic long-chain fatty acylated cellulose and its self-assembled nanoparticles. Polym. Bull. 2012, 69, 389–403. [Google Scholar] [CrossRef]
- Edgar, K.J.; Arnold, K.M.; Blount, W.W.; Lawniczak, J.E.; Lowman, D.W. Synthesis and properties of cellulose acetoacetate. Macromolecules 1995, 28, 4122–4128. [Google Scholar] [CrossRef]
- Yoshida, Y.; Isogai, A. Preparation and characterization of cellulose β -ketoesters prepared by homogeneous reaction with alkylketene dimers: Comparison with cellulose fatty esters. Cellulose 2007, 14, 481–488. [Google Scholar] [CrossRef]
- Yoshida, Y.; Isogai, A. Thermal and liquid crystalline properties of cellulose b-ketoesters prepared by homogeneous reaction with ketene dimmers. Cellulose 2006, 13, 637–645. [Google Scholar] [CrossRef]
- Song, X.; Chen, F.; Liu, F. Preparation and characterization of alkyl ketene dimer (AKD) modified cellulose composite membrane. Carbohydr. Polym. 2012, 88, 417–421. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.F.; Pfeiffer, K.S.; Hussain, M.A. Unconventional cellulose esters: synthesis, characterization. Cellulose 2003, 10, 283–296. [Google Scholar]
- Zarth, C.S.P.; Koschella, A.; Pfeifer, A.; Dorn, S.; Heinze, T. Synthesis and characterization of novel amino cellulose esters. Cellulose 2011, 18, 1315–1325. [Google Scholar] [CrossRef]
- Heinze, T.; Pohl, M.; Schaller, J.; Meister, F. Novel Bulky Esters of Cellulose. Macromol. Biosci. 2007, 7, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Beatriz, A.P.; Ass, B.A.P.; Frollini, E.; Heinze, T. Studies on the Homogeneous Acetylation of Cellulose in the Novel Solvent Dimethyl Sulfoxide/Tetrabutylammonium Fluoride Trihydrate. Macromol. Biosci. 2004, 4, 1008–1013. [Google Scholar]
- Casarano, R.; Nawaz, H.; Possidonio, S.; da Silva, V.C.; El Seoud, O.A. A convenient solvent system for cellulose dissolution and derivatization: Mechanistic aspects of the acylation of the biopolymer in tetraallylammonium fluoride/dimethyl sulfoxide. Carbohyd. Polym. 2011, 86, 1395–1402. [Google Scholar] [CrossRef]
- Hussain, M.A.; Liebert, T.; Heinze, T. Acylation of Cellulose with N,N’-Carbonyldiimidazole-Activated Acids in the Novel Solvent Dimethyl Sulfoxide/Tetrabutylammonium Fluoride. Macromol. Rapid Commun. 2004, 25, 916–920. [Google Scholar] [CrossRef]
- Nagel, M.C.V.; Heinze, T. Esterification of cellulose with acyl-1H-benzotriazole. Polym. Bull. 2010, 65, 873–881. [Google Scholar] [CrossRef]
- Xu, D.; Edgar, J.K. TBAF and cellulose esters: unexpected deacylation with unexpected regioselectivity. Biomacromolecules 2012, 13, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Rahn, K. Cellulose p-toluenesulfonate: A valuable intermediate in cellulose chemistry. Macromol. Symp. 1997, 120, 103–113. [Google Scholar] [CrossRef]
- Heinze, T. Hot Topics in Polysaccharide Chemistry—Selected Examples. Macromol. Symp. 2009, 280, 15–27. [Google Scholar] [CrossRef]
- McCormick, C.L.; Dawsey, T.R.; Newman, J.K. Competitive formation of cellulose p-toluenesulfonate and chlorodeoxycellulose during homogeneous reaction of p-toluenesulfonyl chloride with cellulose in N,N-dimethylacetamide-lithium chloride. Carbohydr. Res. 1990, 208, 183–191. [Google Scholar] [CrossRef]
- Isogai, A.; Ishizu, A.; Nakano, J. Preparation of tri-O-alkylcelluloses by the use of a nonaqueous cellulose solvent and their physical characteristics. J. Appl. Polym. Sci. 1986, 31, 341–352. [Google Scholar] [CrossRef]
- Isogai, A.; Ishizu, A.; Nakano, J. Dissolution mechanism of cellulose in sulfur dioxide-amine-dimethyl sulfoxide. J. Appl. Polym. Sci. 1987, 33, 1283–1290. [Google Scholar] [CrossRef]
- Ramos, L.A.; Frollini, E.; Koschella, A.; Heinze, T. Benzylation of cellulose in the solvent dimethylsulfoxide/tetrabutylammonium fluoride trihydrate. Cellulose 2005, 12, 607–619. [Google Scholar] [CrossRef]
- Pezold-Welcke, K.; Michaelis, N.; Heinze, T. Unconventional cellulose products through nucleophilic displacement reactions. Macromol. Symp. 2009, 280, 72–85. [Google Scholar] [CrossRef]
- Nishimura, H.; Donkai, N.; Miyamoto, T. Preparation and properties of a new type of comb-shaped, amphiphilic cellulose derivative. Cellulose 1997, 4, 89–98. [Google Scholar] [CrossRef]
- Heinze, T.; Lincke, T.; Fenn, D.; Koschella, A. Efficient allylation of cellulose in Dimethyl sulfoxide/tetrabutylammonium fluoride trihydrate. Polym. Bull. 2008, 61, 1–9. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A.; Magdaleno-Maiza, L.; Ulrich, A.S. Nucleophilic displacement reactions on tosyl cellulose by chiral amines. Polym. Bull. 2001, 46, 7–13. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A.; Brackhagen, M.; Engelhardt, J.; Nachtkamp, K. Studies on non-natural deoxyammonium cellulose. Macromol. Symp. 2006, 244, 74–82. [Google Scholar] [CrossRef]
- Liebert, T.; Haensch, C.; Heinze, T. Click Chemistry with Polysaccharides. Macromol. Rapid Commun. 2006, 27, 208–213. [Google Scholar] [CrossRef]
- Fenn, D.; Pohl, M.; Heinze, T. Novel 3-O-propargyl cellulose as a precursor for regioselective functionalization of cellulose. React. Funct. Polym. 2009, 69, 347–352. [Google Scholar] [CrossRef]
- Schumann, K.; Pfeifer, A.; Heinze, T. Novel Cellulose Ethers: Synthesis and Structure Characterization of 3-Mono-O-(30-hydroxypropyl) Cellulose. Macromol. Symp. 2009, 280, 86–94. [Google Scholar] [CrossRef]
- Zabivalova, N.M.; Bochek, A.M.; Vlasova, E.N.; Volchek, B.Z. Preparation of Mixed Cellulose Ethers by the Reaction of Short Flax Fibers and Cotton Linter with Monochloroacetamide. Russ. J. Appl. Chem. 2007, 80, 300–304. [Google Scholar] [CrossRef]
- Zabivalova, N.M.; Bochek, A.M.; Vlasova, E.N.; Volchek, B.Z. Preparation of mixed ethers by reaction of carboxymethyl cellulose with urea and their physicochemical properties. Russ. J. Appl. Chem. 2008, 81, 1622–1629. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterisation of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Sun, R.; Sun, X.F. Succinoylation of sago starch in the N,N-dimethylacetamide/lithium chloride system. Carbohydr. Polym. 2002, 47, 323–330. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Mccormick, C.L. Structopendant Unsaturated Cellulose Esters via Acylation in Homogeneous Lithium Chloride/N,N-Dimethylacetamide Solutions. J. Appl. Polym. Sci. 1997, 66, 293–305. [Google Scholar] [CrossRef]
- Ciacco, G.T.; Liebert, T.F.; Frollini, E.; Heinze, T. Application of the solvent dimethyl sulfoxide/tetrabutyl-ammonium fluoride trihydrate as reaction medium for the homogeneous acylation of Sisal cellulose. Cellulose 2003, 10, 125–132. [Google Scholar] [CrossRef]
- Liebert, T.F.; Heinze, T. Tailored Cellulose Esters: Synthesis and Structure Determination. Biomacromolecules 2005, 6, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ass, B.A.P.; Ciacco, G.T.; Frollini, E. Cellulose acetates from linters and sisal: Correlation between synthesis conditions in DMAc/LiCl and product properties. Bioresour. Technol. 2006, 97, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, A.; Kitaoka, T.; Wariishi, H. Partial Substitution of Cellulose by Ring-Opening Esterification of Cyclic Esters in a Homogeneous System. J. Appl. Polym. Sci. 2006, 102, 4358–4364. [Google Scholar] [CrossRef]
- Ass, B.A.P.; Belgacem, M.N.; Frollini, E. Mercerized linters cellulose: characterization and acetylation in N,N-dimethylacetamide/lithium chloride. Carbohydr. Polym. 2006, 63, 19–29. [Google Scholar] [CrossRef]
- Ciacco, G.T.; Ass, B.A.P.; Ramos, L.A.; Frollini, E. Sisal, sugarcane bagasse and microcrystalline celluloses: Influence of the composition of the solvent system N,N-dimethylacetamide/Lithium chloride on the solubility and acetylation of these polysaccharides. e-Polymers 2008, 22, 1–11. [Google Scholar] [CrossRef]
- Ciacco, G.T.; Morgado, D.L.; Frollini, E.; Possidonio, S.; El Seoud, O.A. Some Aspects of Acetylation of Untreated and Mercerized Sisal Cellulose. J. Braz. Chem. Soc. 2010, 21, 71–77. [Google Scholar] [CrossRef]
- Casarano, R.; Fidale, L.C.; Lucheti, M.C.; Heinze, T.; El Seoud, O.A. Expedient, accurate methods for the determination of the degree of substitution of cellulose carboxylic esters: Application of UV–vis spectroscopy (dye solvatochromism) and FTIR. Carbohydr. Polym. 2011, 83, 1285–1292. [Google Scholar] [CrossRef]
- Liu, H.; Kar, N.; Edgar, J.K. Direct synthesis of cellulose adipate derivatives using adipic anhydride. Cellulose 2012, 19, 1279–1293. [Google Scholar] [CrossRef]
- Glasser, W.G.; Becker, U.; Todd, G.J. Novel cellulose derivatives: Part VI. Preparation and thermal analysis of two novel cellulose esters with fluorine-containing substituents. Carbohydr. Polym. 2000, 42, 393–400. [Google Scholar] [CrossRef]
- Nagel, C.V.M.; Heinze, T. Study about the efficiency of esterification of cellulose under homogeneous condition: dependence on the chain length and solvent. Lenzinger Ber. 2012, 90, 85–92. [Google Scholar]
- Liebert, T.; Hussain, A.M.; Tahir, N.M.; Heinze, T. Synthesis and Characterization of Cellulose α -Lipoates: A Novel Material for Adsorption onto Gold. Polym. Bull. 2006, 57, 857–863. [Google Scholar] [CrossRef]
- Crépya, L.; Miria, V.; Joly, N.; Martina, P.; Lefebvrea, M.J. Effect of side chain length on structure and thermomechanical properties of fully substituted cellulose fatty esters. Carbohydr. Polym. 2011, 83, 1812–1820. [Google Scholar] [CrossRef]
- Devi, S.K.; Mathur, V.K.; Nayak, S.K.; Mohanty, S.; Kumar, M. Homogeneous esterification of cellulose and characterization of cellulose esters. Der Pharma Chemica 2009, 1, 296–303. [Google Scholar]
- McCormick, L.C.; Callais, A.P. Derivatization of cellulose in lithium chloride and N-N-dimethylacetamide solutions. Polymer 1987, 28, 2317–2323. [Google Scholar] [CrossRef]
- Krouit, M.; Granet, R.; Krausz, P. Photobactericidal plastic films based on cellulose esterified by chloroacetate and a cationic porphyrin. Bioorg. Med. Chem. 2008, 16, 10091–10097. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel Biodegradable Superabsorbent Hydrogels Derived from Cotton Cellulose and Succinic Anhydride: Synthesis and Characterization. J. Appl. Polym. Sci. 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, F.; Hou, G. Synthesis and properties of fatty acid esters of cellulose. J. Sci. Ind. Res. 2007, 66, 1019–1024. [Google Scholar]
- Zhou, Q.; Zhang, L.; Minoda, M.; Miyamoto, T. Phase transition of thermosensitive amphiphilc cellulose esters bearing olig (oxyethylenes). Polym. Bull. 2000, 45, 381–388. [Google Scholar] [CrossRef]
- Rahn, K.; Diamantoglou, M.; Klemm, D.; Berghmans, H.; Heinze, T. Homogeneous synthesis of cellulose p-toluenesulfonates in N,N-dimethylacetamide/LiCl solvent system. Angew. Makromol. Chem. 1996, 238, 143–163. [Google Scholar] [CrossRef]
- Heinze, T.; Erler, U.; Heinze, U.; Camacho, J.; Grunnt, U.; Klemm, D. Synthesis and characterization of photosensitive 4,4'-bis(dimethylamino) diphenylmethyl ethers of cellulose. Macromol. Chem. Phys. 1995, 196, 1937–1944. [Google Scholar] [CrossRef]
- Liebert, T.; Heinze, T. Synthesis path versus distribution of functional groups in cellulose ethers. Macromol. Symp. 1998, 130, 271–283. [Google Scholar] [CrossRef]
- Liebert, T.F.; Heinze, T. Exploitation of Reactivity and Selectivity in Cellulose Functionalization Using Unconventional Media for the Design of Products Showing New Superstructures. Biomacromolecules 2001, 2, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T. Carboxymethyl ethers of cellulose and starch—A review. Khimiya Rastitel’nogo Syr’ya 2005, 3, 13–29. [Google Scholar] [CrossRef]
- Ramos, L.A.; Frollini, E.; Heinze, T. Carboxymethylation of cellulose in the new solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Carbohydr. Polym. 2005, 60, 259–267. [Google Scholar] [CrossRef]
- Koschella, A.; Heinze, T.; Klemm, D. First Synthesis of 3-O-Functionalized Cellulose Ethers via 2,6-Di-O-Protected Silyl Cellulose. Macromol. Biosci. 2001, 1, 49–54. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A. Water-soluble 3-O-(2-methoxyethyl) cellulose synthesis and characterization. Carbohydr. Res. 2008, 343, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, H.; Koschella, A.; Mikawa, Y.; Nakatsubo, F.; Heinze, T.; Klemm, D. Syntheses and Comparison of 2,6-Di-O-methyl Celluloses from Natural and Synthetic Celluloses. Macromol. Biosci. 2008, 8, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; Koehler, S.; Liebert, T.; Heinze, T. Pure Cellulose Nanoparticles fromTrimethylsilyl Cellulose. Macromol. Symp. 2010, 294, 96–106. [Google Scholar] [CrossRef]
- Heinze, T.; Wang, Y.; Koschella, A.; Sullo, A.; Foster, J.T. Mixed 3-mono, O-alkyl cellulose: Synthesis, structure characterization and thermal properties. Carbohydr. Polym. 2012, 90, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Petzold, K.; Koschella, A.; Klemm, D.; Heublein, B. Silylation of cellulose and starch—Selectivity, structure analysis, and subsequent reactions. Cellulose 2003, 10, 251–269. [Google Scholar] [CrossRef]
- Ikeda, I.; Washino, K.; Maeda, Y. Graft polymerization of cyclic compounds on cellulose dissolved in tetrabutylammonium fluoride/dimethyl sulfoxide. Sen’I Gakkaishi 2003, 59, 110–114. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zheng, W.; Zhu, J. Regenerated cellulose/graphene nanocomposite films prepared in DMAC/LiCl solution. Carbohydr. Polym. 2012, 88, 26–30. [Google Scholar] [CrossRef]
- Williamson, L.S.; McCormick, L.C. Cellulose derivatives synthesized via isocyanate and activated ester pathways in homogeneous solutions of lithium chloride/N,N-dimethylacetamide. J. Macromol. Sci. A Pure Appl. Chem. 1998, 12, 1915–1927. [Google Scholar] [CrossRef]
- Liebert, T.; Nagel, M.C.V.; Jordan, T.; Heft, A.; Gruenler, B.; Heinze, T. Pure, Transparent-Melting Starch Esters: Synthesis and Characterization. Macromol. Rapid Commun. 2011, 32, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.; Charlet, G. Chitin fractionation and characterization in N,N-dimethylacetamide/ lithium chloride solvent system. Carbohydr. Polym. 2002, 50, 363–370. [Google Scholar] [CrossRef]
- Zhong, C.; Cooper, A.; Kapetanovic, A.; Fang, Z.; Zhang, M.; Rolandi, M. A facile bottom-up route to self-assembled biogenic chitin nanofibers. Soft Matter 2010, 6, 5298–5301. [Google Scholar] [CrossRef]
- Pestov, A.V.; Koryakova, O.V.; Leonidov, I.I.; Yatluk, Y.G. Gel-Synthesis, Structure, and Properties of Sulfur-containing Chitosan Derivatives. Russ. J. Appl. Chem. 2010, 83, 787–794. [Google Scholar] [CrossRef]
- Teramoto, Y.; Miyata, T.; Nishio, Y. Dual Mesomorphic Assemblage of Chitin Normal Acylates and Rapid Enthalpy Relaxation of Their Side Chains. Biomacromolecules 2006, 7, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, G.; Uzun, I.; Guezel, F. Adsorption of some highly toxic dyestuffs from aqueous solution by chitin and its synthesized derivatives. Desalination 2009, 249, 1115–1123. [Google Scholar] [CrossRef]
- Sugimoto, M.; Kawahara, M.; Teramoto, Y.; Nishio, Y. Synthesis of acyl chitin derivatives and miscibility characterization of their blends with poly (ε-caprolactone). Carbohydr. Polym. 2010, 79, 948–954. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, X.P.; Liu, X.F.; Guan, Y.L.; Yao, K.D. Study on antibacterial O-carboxy methylated chitosan/cellulose blend film from LiCl/N,N-dimethylacetamide solution. Polymer 2002, 43, 1541–1547. [Google Scholar] [CrossRef]
- Grote, C.; Heinze, T. Starch derivatives of high degree of functionalization 11: studies on alternative acylation of starch with long-chain fatty acids homogeneously in N,N-dimethyl acetamide/LiCl. Cellulose 2005, 12, 435–444. [Google Scholar] [CrossRef]
- Duan, W.-G.; Fang, H.-X.; Ma, H.-G.; Li, G.-H.; Cen, B. Microwave-assisted synthesis of maleated rosin-cassava starch esters. Lin Chan Hua Xue Yu Gong Ye 2009, 29, 16–22. [Google Scholar]
- Einfeldt, L.; Petzold, K.; Gunther, W.; Stein, A.; Kussler, M.; Klemm, D. Preparative and 1H-NMR Investigation on Regioselective Silylation of Starch Dissolved in Dimethyl Sulfoxide. Macromol. Biosci. 2001, 1, 341–347. [Google Scholar] [CrossRef]
- Petzold, K.; Einfeldt, L.; Guenther, W.; Stein, A.; Klemm, D. Regioselective Functionalization of Starch: Synthesis and 1H-NMR Characterization of 6-O-Silyl Ethers. Biomacromolecules 2001, 2, 965–969. [Google Scholar] [CrossRef] [PubMed]




























| Starch Source | % Amylose | % Amylopectin |
|---|---|---|
| Rice (Japonica) | 17.5 | 82.5 |
| Wheat (Asw) | 21.7 | 78.3 |
| Barley (Bomi) | 27.5 | 72.5 |
| Maize (Normal) | 21.5 | 78.5 |
| Maize (Hylon 7) | 58.6 | 41.4 |
| Water Chestnut | 23.3 | 76.7 |
| Chestnut | 19.6 | 80.4 |
| Sago | 24.3 | 75.7 |
| Lotus root | 15.9 | 84.1 |
| Kuzu root | 21 | 79 |
| Sweet Potato | 18.9 | 81.1 |
| Yam | 22 | 78 |
| Lentil | 29–45 | 71–54 |
| Tapioca | 16.7 | 83.3 |
| Arrowroot | 25.6 | 74.4 |
| Edible Canna | 22.2 | 77.8 |
| Potato | 21 | 79 |
| Waxy Maize | <1 | >99 |
| Corn | 24–28 | 75 |
| Waxy Corn | 0 | 100 |
| Entry | Polysaccharide; DP; Ic | Dissolution solvent system | Dissolution conditions (temperature, heating time) | Techniques employed to study dissolution | Reference |
|---|---|---|---|---|---|
| 1 | MCC | 5–8% LiCl/DMAC | 150 °C, 2 h | GPC | [76] |
| 2 | Cotton Linters; 1776; 0.50 Sulphite pulp; 728; 0.42 | 3.5–8% LiCl/DMAC; EWNN | 2–35 min; RT | SEM; TEM | [49] |
| 3 | Kraft pulps | 8% LiCl/DMAC | 4 °C; 5 d | 13C-CP/NMR | [77] |
| 4 | MCC; 280 Sulphite pulp; 1020 | 7.5% LiCl/DMAC | 130 °C; 1 h | Solvatochromic technique, UV/VIS | [64] |
| 5 | MCC; 155; 0.81 Bagasse; 780; 0.82 | 8.3% LiCl/DMAC | 155 °C; 1 h | FTIR, X-Ray, SEM | [56] |
| 6 | MCC; 285 Buckeye; 1360 | 1–9% LiCl/DMAC | RT; overnight | LS | [78] |
| 7 | Cotton linters | 8% LiCl/DMAC | <60 °C; RT | Viscometry | [79] |
| 8 | MCC; 440 | 5% LiCl/DMAC | 100 °C; overnight | Fiber spinning method | [80] |
| 9 | Wood cellulose | 8% LiCl/DMAC | 80 °C; 4 h | SEC, MALLS | [81] |
| 10 | Cotton fiber | 11% LiCl/DMAC | RT; overnight | SEC | [82] |
| 11 | Sulfite pulp | 1–9% LiCl/DMAC | 25 °C; 3 d | Phase diagram | [73] |
| 12 | Sulfite pulp | 8% LiCl/DMAC | RT | WAXD, SAXS | [83] |
| 13 | Dissolving pulp | 8% LiCl/DMAC | RT; few days | LS, SAXS | [84] |
| 14 | Whatman No.1 paper | 8% LiCl/DMAC | RT; 15 h | SEC | [85] |
| 15 | Tunicate cellulose | 8% LiCl/DMI | RT 6–9 months | LS, Viscometry | [86] |
| 16 | Whatman No.1 paper | 8% LiCl/DMAC | RT; 24 h | SEC, MALS,DRI | [87] |
| 17 | MCC; 126; 0.83 Cotton linter; 400; 0.80 Sisal; 642; 0.67 | 7.4% LiCl/DMAC | 150 °C; 1.5 h | X-Ray Diffraction, SEM, | [25] |
| 19 | Cotton linter | 8% LiCl/DMAC | RT; 1 month | SLS | [88] |
| 20 | MCC | 8% LiCl/DMAC | 20 °C; 1, 4, 8, 48 h | WAXS, 13C-NMR | [89] |
| 21 | Fibrous cellulose 390 | 7% LiCl/DMAC | 80 °C; 0.75 h | WAXD, FTIR | [90] |
| 23 | MCC; 332 | 7.5% LiCl/DMAC; 3.5% TBAF/DMSO | 130 °C; 2 h 80 °C; 2 h | FTIR, 1H,13C-NMR | [31] |
| 24 | Soft wood sulfite pulp | 8% LiCl/DMAC | RT; few h | SAXS, FTIR, 13C-NMR | [91] |
| 25 | Amorphous cellulose | 1.5% LiCl/DMAC | 160 °C; 20 °C, overnight | SAXS, AFM, | [92] |
| 26 | MCC | 10% LiCl/DMAC | 150 °C; 2 h, 150 °C; 3 min, microwave | 1H-NMR, DLS, Viscometry | [93] |
| 27 | MCC; 163 | 8% LiCl/DMAC | RT; 3 min | WAXS, Density measurement | [94] |
| 28 | Bacterial cellulose | 8% LiCl/DMAC | RT; 10, 15, 20, 40, 60 min. | X-Ray, SEM | [95] |
| 29 | MCC; 210–270 | 1% TBAF/DMSO | 60 °C; 20 min, | 19F,1H-NMR | [74] |
| 30 | Beach and spruce milled wood | 6% LiCl/DMSO; 6% LiCl/DMAC | RT; 2 h | X-Ray diffraction | [96] |
| 31 | Cotton pulp; 4500 | 8% LiCl/DMAC | 155 °C; 1 h | SEM | [97] |
| 32 | Cotton | 8% LiCl/DMAC | 155 °C; 1 h | SEM, UV-vis, X-Ray | [98] |
| 33 | Cotton | 14% TBAF/DMSO | 60 °C | 1H-NMR, FTIR | [99] |
| 34 | MCC | 9% LiCl/DMAC | RT; 1 h | X-Ray, SEM, ATR-FTIR, | [100] |
| 35 | MCC; 332 | 10% TBAF/DMSO | 60 °C; 1 h | 19F, 1H-NMR | [101] |
| 36 | Beech, eucalyptus | 8% LiCl/DMI | RT; 3–4 d | SEC, LS | [102] |
| 37 | Cotton | 9% LiCl/DMAC | 155 °C; 4 h | Laser Doppler vibrometer | [103] |
| 38 | MCC | 9% LiCl/DMAC | ------- | SEC, SEM | [104] |
| 39 | Sisal; 642; 0.67 Cotton linters, 400; 0.80 | 8.3% LiCl/DMAC | 150 °C; 1.5 h | X-Ray, Viscometry | [105] |
| 40 | Canola straw | 8% LiCl/DMAC | RT; 5–120 min | X-Ray | [106] |
| 41 | Kraft pulp | 8% LiCl/DMAC; 16.25% TBAF/DMSO | 4 °C; 5 d | SEC, 13C-NMR | [107] |
| 42 | Cellulose membrane | 8.1% LiCl/DMAC | 100 °C; 6 h | HPLC | [108] |
| 43 | Cotton linters Softwood kraft pulp | 9% LiCl/DMAC | 40 °C; 0.5–120 h | GPC, SEM | [109] |
| 44 | Cellulose powder | 10% LiCl/DMAC | 100 °C; 7.5 h | SAXS, SEM | [110] |
| 45 | Kraft bleached pulp | 8.5% LiCl/DMAC; 8.5% LiCl/NMP | ------- | 13C-NMR | [69] |
| 46 | Cellulose | 5–10% LiCl/DMAC | ------- | 1H,13C, 7Li-NMR | [111] |
| 47 | Avicel; 170 | LiCl/DMAC | RT; 1 h | 7Li-NMR | [68] |
| 48 | Regenerated cellulose; 174 | 5–10% LiCl/DMSO | RT; 24 h | FTIR, 13C-NMR | [112] |
| 50 | oligosaccharides | 0.01% LiCl/DMAC | 100 °C; 1 h | ESI-TS, FAB | [113] |
| 54 | Wood cellulose | 10% LiCl/DMAC | RT; 6 h | FTIR | [114] |
| 55 | MCC; 280 | 9% LiCl/DMAC | 80 °C; 1.5 h | FTIR, WAXD, SEM | [115] |
| 56 | MCC; M-cotton; M-sisal | 6% LiCl/DMAC | 110 °C; 4 h | Viscometry, SLS, 1H-NMR | [71] |
| 57 | Sulfite softwood | 8% LiCl/DMAC | 35–40 °C; 1 day | SEC | [116] |
| 58 | Beech sulfite pulp | 9% LiCl/DMAC | 85–125 °C | GPC | [117] |
| 59 | Sulfite pulp; 1500 | 8% LiCl/DMAC | RT; 2 d | SEC | [118] |
| 60 | pulp | 9% LiCl/DMAC | 85–125 °C | 1H-NMR | [119] |
| 61 | Cotton linter | 8% LiCl/DMAC | RT; 1 month | SLS, DLS | [120] |
| 62 | Cotton cellulose | 8% LiCl/DMAC | RT; 2–3 months | SLS, DLS | [121] |
| 63 | Dissolving pulp | 8% LiCl/DMAC | RT; few days | SAXS | [122] |
| 64 | MCC; 280 | 9% LiCl/DMAC | 150 °C; 1 h | SEM | [123] |
| 65 | Cotton linters | 5.3% LiCl/DMAC | 80 °C; 1 h | Viscometry | [124] |
| 66 | Hard wood kraft pulp | 6–10% LiCl/DMAC | 4 °C; 5 d | SEC | [125] |
| Entry | Cellulose type; DP; Ic | Derivatizing agent | Reaction conditions: Ratio derivatizing agent/AGU; Temp. °C; Reaction time, h | DS range | Solvent used | Study Techniques | Reference |
|---|---|---|---|---|---|---|---|
| Esters of carboxylic- and sulfonic acids | |||||||
| 1 | MCC, hardwood pulp | Diketene; Butyric anhydride | 3:1; 110 °C; 40 min. | 0.90, 0.30 | 5% LiCl/DMAC, DMSO, NMP | GPC, DSC, 1H-NMR, 13C-NMR | [136] |
| 2 | MCC | Unsat. Carboxylic acids/DCC; acid anhydr./DMAP | 25 °C; 48 h | 0.25–0.55 | 9% LiCl/DMAC | FTIR, 1H-NMR | [164] |
| 3 | MCC; 0.79 | Ac2O | 1–9:1; 110 °C; 4 h | 0.9–2.8 | 6% LiCl/DMAC | X-Ray, 13C-NMR | [58] |
| 4 | cellulose | Fatty acid chlorides, Ac2O | 2:1; 130 °C; 5 h | 2.9–3.0 | 9% LiCl/DMAC | 1H-NMR, GC-MS, | [128] |
| 5 | MCC; 260 | Acyl chlorides, TsCl | 5:1; 80 °C; 2 h | 2.96 | 7.5% LiCl/DMAC | 1H-NMR, FTIR | [138] |
| 6 | Avicel; 330, sisal; 650 | Ac2O; stearic anhyd; vinyl acetate | 11:1; 60 °C; 3 h | 1.20 | 11% TBAF/ DMSO | 1H-NMR, HPLC | [165] |
| 7 | Cotton linter; 440 | Ac2O | 2–13:1, 60 °C; 3–24 h | 0.43–2.77 | 9% TBAF/ DMSO | FTIR, 1H-NMR | [141] |
| 8 | MCC; 260 | Ac2O; stearic acid; adamantane carboxylic acids; CDI | 3:1; 80 °C; 24 h | 0.5–1.9 | 10% TBAF/ DMSO | FTIR, 1H-NMR | [143] |
| 9 | MCC; 280 | Carboxylic acids; CDI | 5:1:5; 60 °C; 24 h, | 2.5 | 7.5% LiCl/DMAC, 10% TBAF/DMSO | 1D, 2D-NMR | [166] |
| 10 | Sisal; 650; 0.54 cotton linter; 410; 0.77 | Ac2O | 2:1; 110 °C; 4 h | 1.5 | 5–7% LiCl/DMAC | SEC, 1H-NMR | [167] |
| 11 | Whatman CF-1 cellulose powder, 200 | Cyclic lactones | 4:1; 128 °C; 12 h | 0.7 | 8% LiCl/DMAC | FT-Raman, 13C-NMR | [168] |
| 12 | Mercerized Cotton linter | Ac2O, NaOH | 3:1; 110 °C; 1–5 h | 1.1–2.2 | 6% LiCl/DMAC | SEM, 1H-NMR | [169] |
| 13 | MCC; 280 | Carboxylic acids, CDI | 3:1:3; 80 °C; 24–36 h | 0.7 | 16.16% TBAF/DMSO; 5–10% LiCl/DMAC | 1H-NMR | [140] |
| 14 | MCC; sisal | Ac2O | 3:1; 110 °C; 1–4 h | 1.6 | 5–9% LiCl/DMAC | 1H-NMR, X-Ray | [170] |
| 15 | MCC; 260 | Acyl-1H-benzotriazole | 3:1; 60 °C; 3 h | 1.07–1.89 | 11% TBAF/DMSO | FTIR, 1H-NMR | [144] |
| 16 | MCC; 100–200 | Ac2O | 6:1; 110 °C | 1.5 | 6% LiCl/DMAC | SLS, 1H-NMR | [171] |
| 17 | MCC; cotton linter; sisal | Ac2O | 0.5–6:1; 110 °C; 4 h | 2.7 | 6% LiCl/DMAC | SLS, 1H-NMR | [69] |
| 18 | MCC | Acid anhydrides; diketene | 1–3:1; 18 h; RT | 1–2.8 | 8% LiCl/DMAC | X-Ray diffraction, 13C-NMR | [32] |
| 19 | MCC | Acid anhydrides | 1–4.5:1; 18 h; 60 °C | 1–2.8 | 8% LiCl/DMAC | Viscometry, X-Ray Diffraction | [33] |
| 20 | MCC; Cotton linters | Ac2O | 0.5–12:1; 4 h, 110 °C | 0.2–2.8 | 5–8% LiCl/DMAC | 13C-NMR | [30] |
| 21 | MCC; 175; Sisal; 800 | acid anhydride | 4:1; 18 h; 60 °C | 2.0 | 8% LiCl/DMAC | UV-Vis, FTIR | [172] |
| 22 | MCC; 175; eucalyptus; 1049 | Acid anhydrides | 6–13:1; 3 h; 60–100 °C | 1.6–2.4 | 9% TBAF/DMSO | Viscometry, 1H-NMR | [142] |
| 23 | MCC; hard wood pulp | Butyric anhydride; diketene | 3:1; 30–40 min; 110 °C | 0.3–2.9 | 7% LiCl/DMAC | GPC, 1H,13C-NMR | [134] |
| 24 | MCC | Adipic anhydride | 1–3:1; 2–20 h; 60–90 °C | 2.1–2.6 | 5% LiCl/DMAC; 5% LiCl/DMI | 1H-NMR, FTIR, SEC | [173] |
| 25 | Whatman CF-11; 190 | Chloroacetic acid; TsCl | 1:1; 24 h; 40–50 °C | 1.5–2.6 | 9% LiCl/DMAC | 1H, 19F-NMR | [174] |
| 26 | MCC; 300; Spruce sulfite pulp; 650 | Acetic anhydride; vinyl carboxylates | 2.3–10:1; 70 h; 40 °C | 0.8–2.7 | 5% TBAF/DMSO | 1H, 13C-NMR FTIR | [31] |
| 27 | Avicel; 260 | Acid anhydrides, carboxylic acids, CDI | 3:1; 3 h; 60 °C | 1.18 | 7.5% LiCl/DMAC | FTIR, 1H-NMR | [175] |
| 28 | MCC; 280 | α-lipoic acid, TsCl or CDI | 3:1; 16 h 60 °C | 1.45 | LiCl/DMAC | FTIR, 1H-NMR | [176] |
| 29 | MCC; 150 | Acid chlorides, DMAP | 5–8:1; 3 h; 80 °C | 2.8 | 6.7% LiCl/DMAC | FTIR, 1H-NMR; DSC, WAXS | [177] |
| 30 | Cellulose; 141 | Ac2O | 20:1; 2–24 h; 28–70 °C | 1.65–2.85 | 1.6% LiCl/DMAC | Viscometry, FTIR | [178] |
| 31 | Cellulose | Acid chlorides | 1:1; 8 h; 25 °C | 2.4 | 9% LiCl/DMAC | Elemental analysis | [179] |
| 32 | Cellulose | Chloroacetyl chloride | 6:1; 2 h; RT | 2.8 | 10% LiCl/DMAC | FTIR, 1H-NMR | [180] |
| 33 | MCC Whatman CF-11 | Carboxylic acids; TsCl | 2: 24 h; 50 °C | 2.8–2.9 | 4% LiCl/DMAC | 1H-NMR, DSC | [127] |
| 34 | Cotton | Succinic anhydride, DMAP | 20:1; 24 h; RT | 2.5–2.6 | 8% LiCl/NMP; 15% TBAF/DMSO | Titration | [181] |
| 35 | MCC; 280 | Acid chlorides | 6:1; 24 h; RT | 1.57 | 2.5% LiCl/DMAC | FTIR, WAXD, SEM | [182] |
| 36 | Whatman CF-11 | Acid chloride, acetic anhydride pyridine | 4 h; 60 °C | 0.4–3.0 | 10% LiCl/DMAC | 13C-NMR, UV-Vis | [183] |
| 37 | MCC; 280–5100 | TsCl | 0.6–9:1; 130 °C; 2 h | 0.4–2.3 | 5% LiCl/DMAC | FTIR, 13C-NMR | [184] |
| Nonionic and ionic ethers | |||||||
| 38 | MCC | 4,4'-Bis(dimethylamino)-diphenylmethyl chloride | 2:1; 50 °C; 24 h | 0.54–1.05 | DMAC, DMSO | UV, 13C-NMR | [185] |
| 39 | MCC; 280; Sulfite pulp; 680 Cotton linters; 1350 | ClCH2CO2Na/NaOH | 5:1; 70 °C; 48 h | 2.07 | 1.7% LiCl/DMAC | HPLC, 1H, 13C-NMR | [186] |
| 40 | MCC; 330; Dissolving pulp; 950 | ClCH2CO2Na/NaOH | 10:5:1; 0.5–4 h, 70 °C | 1.82–2.09 | 18.5% TBAF/DMSO; NMMNO/DMSO | 1H, 13C-NMR, HPLC | [187] |
| 41 | Sisal; 574; Cotton linters; 400 | Benzyl chloride, NaOH | 3:1; 4 h; 70 °C | 0.4–2.85 | 9% DMSO/TBAF | FTIR, 1H,13C-NMR, SEC | [151] |
| 42 | MCC | ClCH2CO2Na/NaOH | 5:1; 48 h; 70 °C | 2.07 | LiCl/DMAC | FTIR, 1H-NMR | [61] |
| 43 | Sulfite pulp; 504 | Allyl chloride, NaOH | 36:1:36; 50 °C; 3 d | 2.7 | 10.9% TBAF/ DMSO | 1H-NMR, FTIR | [154] |
| 44 | MCC | ClCH2CO2Na/NaOH | 2:1; 48 h; 70 °C | 1.88 | LiCl/DMAC | FTIR, 1H-NMR | [188] |
| 45 | Sisal; 640; 0.64; Linter; 400; 0.73 | ClCH2CO2Na/NaOH | 5:1:10; 70 °C; 4 h | 2.17 | 9% TBAF/DMSO | SEC, HPLC, 1H-NMR | [189] |
| 46 | MCC | TDMSCl, Imidazole | 4:1; 100 °C; 24 h | 2.0 | 5% LiCl/DMAC | 1H, 13C, COSY, HMQC, NMR | [190] |
| 47 | MCC; 419; Sulfite pulp; 560 | TDMSCl, Imidazole | 4:1:5; 100 °C; 24 h | 1.98 | 8.4% LiCl/DMAC | 1H-NMR, FTIR | [191] |
| 48 | MCC; 280 | TDMSCl; allyl chloride; methyl iodide; benzyl chloride | RT; 42 h | 1.92 | LiCl/DMAC; TBAF/DMSO | 1H,13C-NMR, UV-VIS, GPC | [192] |
| 49 | MCC | HMDS, TDMSCl | 0.1:1:2; 80 °C; 1 h | 2.89 | LiCl/DMAC | FTIR, 1H-NMR | [193] |
| 50 | MCC; 117 | TDMSCl, Imidazole | 4.1:1; 24 h; 100 °C | 2.06 | 7.8% LiCl/DMAC | FTIR, 1H-NMR | [194] |
| 51 | MCC | HMDS | 8:1; 1 h; 80 °C | 2.7–2.9 | LiCl/DMAC | 1H-NMR | [195] |
| Miscellaneous | |||||||
| 52 | Avicel; 100–300 | Lactones; N-carboxy α-amino acid anhydrides | 2:1; 60 °C; 4 h | 15% TBAF/DMSO | FTIR, GPC | [196] | |
| 53 | Cotton linter; 640 | Graphene | 85 °C; 0.5 h | 9% LiCl/DMAC | SEM, TGA | [197] | |
| 54 | Cellulose | Phenyl isocyanate, pyridine | 2.7:1; 12 h; RT | 2.6 | 9% LiCl/DMAC | FTIR | [198] |
| 55 | MCC; 156; 290 | ε-Caprolactam, N-methyl- ε-caprolactam, TsCl | 5:1; 5 h; RT | 0.12–1.17 | 9% LiCl/NMP | FTIR, 1H-NMR | [137] |
| Entry | Polysaccharide | Dissolution conditions, temperature and time | Derivatizing agent | Reactions conditions Ratio derivatizing agent/AGU/cat.; T, °C; Reaction time, h | Yield; DS | Dissolution solvent | Study Techniques | Reference |
| Chitin/chitosan | ||||||||
| 1 | Chitin | RT, 1.5 h | ---- | ------ | ----- | 5% LiCl/DMAC | SEC, MALLS | [200] |
| 2 | Chitin | ----- | ------ | ------ | ----- | 0.01–0.2% LiCl/DMAC | FTIR | [201] |
| 3 | Chitin, squid pens | RT, 120 h | ----- | ------ | ----- | 5% LiCl/DMAC | Viscometry | [13] |
| 4 | Chitosan | ------ | ------ | 6:1; 50 °C; 24 h | 0.31 | 2.3% HCl/LiSCN | IR | [202] |
| 5 | Chitin, crab shell | RT, 5 d | Alkanoic acid, p-TsCl, Pyridine | 8:1:8; 50 °C; 100 h | 1.7–1.9 | 9% LiCl/DMAC | WAXD; FTIR, 1H-NMR | [203] |
| 6 | Chitin | RT, 3 h | Cyclic acid anhydrides | RT; 24 h | ----- | 5% LiCl/DMAC | Kinetic study | [204] |
| 7 | Chitin, crab shell | RT, 5 d | Acid chlorides, TEA | 28:1; 50 °C; 6–48 h | 0.97–1.77 | 7% LiCl/DMAC | GPC, 1H-NMR, FTIR | [205] |
| 8 | Chitin | ------ | Methacrylic acid, DCC, DMAP | 6:1:6; RT; 48 h | ----- | 5% LiCl/DMAC | FTIR, NMR, SEM | [17] |
| 9 | Chitosan | 150 °C, 0.5 h | ClCH2CO2Na/NaOH | ---- | ---- | 9% LiCl/DMAC | FTIR, SEM, WAXS, | [206] |
| Starch | ||||||||
| 10 | Corn amylose | 120 °C, 5 min | ------ | ------- | ----- | 3% LiCl/DMAC | LS | [6] |
| 11 | Lign°Cellulose | ----- | CrCl2 | 6:1; 100 °C; 5 h | ---- | 10% LiCl/DMAC | HPLC, IEC | [1] |
| 12 | Potato starch | 80 °C, 20 min | Acyl chlorides | 3:1:3; 80 °C; 0.5 h | 0.3–3 | 0.6% LiCl/DMAC | FTIR-Elemental analysis | [162] |
| 13 | Sago starch | ------- | Succinic anhydride DMAP, Pyridine | 3:1:3; 105 °C; 0.5–3 h | 0.14–1.54 | --------- | FTIR, 13C-NMR | [163] |
| 14 | Potato starch | 100 °C, 45 min | Fatty acid chlorides, CDI | 1:1; 100 °C; 6 h | 2.17 | 2% LiCl/DMAC | FTIR, 1H, 13C-NMR | [207] |
| 15 | Potato starch | 120 °C, 2 h | Palmitoyl chloride, pyridine | 4.5:1:5; 100 °C; 6 h | 2.63 | 2% LiCl/DMAC | FTIR, 1H, 13C-NMR | [199] |
| 16 | Cassava starch | ------ | Maleopimaric acid chloride Maleopimaric acid, pyridine | 115 °C; 2 h | 0.11–0.17 | DMF/pyridine; Microwave | 1H, 13C-NMR, FTIR | [208] |
| 17 | Maize starch | 80 °C, 2 h | TDMSCl, Pyridine | 0.6–6:1; 20 °C; 20 h | 1.8 | DMSO | 1H-NMR | [209] |
| 18 | Potato starch | −20 °C, 1–2 h, 20 °C, 24 h, | TDMSCl/NMP | 1.6–4:1; 20 °C; 24 h | 1.0 | 12–19% NMP/ammonia | 1H-NMR | [210] |
| 19 | Amylose, amylopectin | ----- | 1. NaN3, 2. PPh3, CBr4 | 10:1; 100 °C; 1 h | ------ | 8% LiCl, LiBr/DMF | IR, 1H, 13C-NMR | [14] |
© 2013 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El Seoud, O.A.; Nawaz, H.; Arêas, E.P.G. Chemistry and Applications of Polysaccharide Solutions in Strong Electrolytes/Dipolar Aprotic Solvents: An Overview. Molecules 2013, 18, 1270-1313. https://doi.org/10.3390/molecules18011270
El Seoud OA, Nawaz H, Arêas EPG. Chemistry and Applications of Polysaccharide Solutions in Strong Electrolytes/Dipolar Aprotic Solvents: An Overview. Molecules. 2013; 18(1):1270-1313. https://doi.org/10.3390/molecules18011270
Chicago/Turabian StyleEl Seoud, Omar A., Haq Nawaz, and Elizabeth P. G. Arêas. 2013. "Chemistry and Applications of Polysaccharide Solutions in Strong Electrolytes/Dipolar Aprotic Solvents: An Overview" Molecules 18, no. 1: 1270-1313. https://doi.org/10.3390/molecules18011270
APA StyleEl Seoud, O. A., Nawaz, H., & Arêas, E. P. G. (2013). Chemistry and Applications of Polysaccharide Solutions in Strong Electrolytes/Dipolar Aprotic Solvents: An Overview. Molecules, 18(1), 1270-1313. https://doi.org/10.3390/molecules18011270