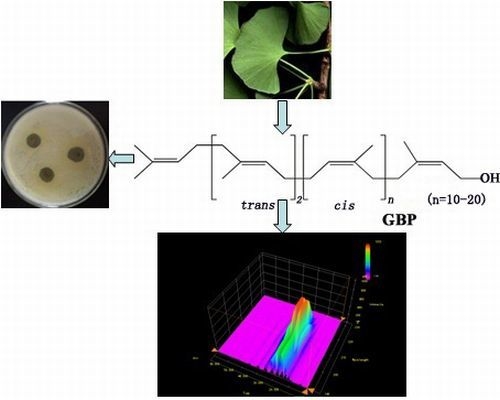
Antibacterial/Antifungal Activity and Synergistic Interactions between Polyprenols and Other Lipids Isolated from Ginkgo Biloba L. Leaves
Abstract
:1. Introduction

2. Results and Discussion
2.1. Structure Determination of Separated Compounds



2.2. Antibacterial and Antifungal Activity of GBL Lipids
| Samples ▲ | Diameter of the inhibition halos (mm) ± SEM, n = 3 | |||||
|---|---|---|---|---|---|---|
| S. enterica | S. aureus | A. niger | ||||
| S1 | 15.1 ± 0.1 a | 16.9 ± 0.1 a | 14.4 ± 0.1 a | |||
| S2 | 12.3 ± 0.1 b | 12.8 ± 0.1 b | 0.0 | |||
| S3 | 14.8 ± 0.1 a | 14.8 ± 0.1 c | 12.9 ± 0.1 b | |||
| S4 | 16.0 ± 0.1 c | 16.8 ± 0.1 a | 15.0 ± 0.1 c | |||
| GBP | 13.5 ± 0.1 d | 13.8 ± 0.1 d | 13.4 ± 0.1 d | |||
| C1 | 9.9 ± 0.1 e | 13.4 ± 0.1 e | 10.3 ± 0.1 e | |||
| C2 | 17.4 ± 0.1 f | 20.1 ± 0.1 f | 16.2 ± 0.1 f | |||
| C3 | 16.1 ± 0.1 c | 14.9 ± 0.1 c | 17.9 ± 0.1 g | |||
| C4 | 10.9 ± 0.1 g | 11.7 ± 0.1 g | 0.0 | |||
| C5 | 12.1 ± 0.1 b | 14.1 ± 0.1 d | 0.0 | |||
| C6 | 7.7 ± 0.1 h | 9.2 ± 0.1 h | 0.0 | |||
| C7 | 8.1 ± 0.1 h | 10.0 ± 0.1 i | 0.0 | |||
| C8 | 11.1 ± 0.1 g | 12.7 ± 0.1 b | 0.0 | |||
| C1:GBP (1:1, wt/wt) | 16.3 ± 0.1 *c | 11.7 Δ | 17.1 ± 0.1 *a | 13.6 Δ | 14.1 ± 0.1 *a | 11.8 Δ |
| C2:GBP (1:1, wt/wt) | 16.2 ± 0.1 *c | 15.4 Δ | 18.3 ± 0.1 *j | 17.0 Δ | 15.3 ± 0.1 c | 14.8 Δ |
| C3:GBP (1:1, wt/wt) | 15.1 ± 0.1 a | 14.8 Δ | 15.2 ± 0.1 *c | 14.4 Δ | 15.0 ± 0.1 c | 15.6 Δ |
| C4:GBP (1:1, wt/wt) | 12.9 ± 0.1 i | 12.2 Δ | 13.1 ± 0.1 b | 12.8 Δ | 7.2 ± 0.1 h | 6.7 Δ |
| C5:GBP (1:1, wt/wt) | 14.8 ± 0.1 *a | 12.8 Δ | 16.3 ± 0.1 *k | 14.0 Δ | 10.4 ± 0.1 *e | 6.7 Δ |
| C6:GBP (1:1, wt/wt) | 10.2 ± 0.1 e | 10.6 Δ | 10.2 ± 0.1 i | 11.5 Δ | 8.3 ± 0.1 *i | 6.7 Δ |
| C7:GBP (1:1, wt/wt) | 10.1 ± 0.1 e | 10.8 Δ | 10.2 ± 0.1 i | 11.9 Δ | 7.0 ± 0.1 h | 6.7 Δ |
| C8:GBP (1:1, wt/wt) | 9.0 ± 0.1 j | 12.3 Δ | 9.1 ± 0.1 h | 13.2 Δ | 6.1 ± 0.1 j | 6.7 Δ |
| MN | 0.0 | 20.4 ± 0.1 | 20.9 ± 0.4 | |||
| GS | 20.2 ± 0.3 | 20.1 ± 0.5 | 0.0 | |||
| Samples | MIC, MBC and MFC values (μg/mL) | |||||
|---|---|---|---|---|---|---|
| S. enterica | S. aureus | A. niger | ||||
| S1 | 15.6 | 62.5 * | 15.6 | 62.5 * | 31.3 | 125 ** |
| S2 | 62.5 | 125 * | 62.5 | 125 * | / | / |
| S3 | 31.3 | 125 * | 31.3 | 125 * | 31.3 | 125 ** |
| S4 | 31.3 | 62.5 * | 15.6 | 62.5 * | 31.3 | 62.5 ** |
| GBP | 31.3 | 125 * | 31.3 | 125 * | 31.3 | 125 ** |
| C1 | 31.3 | 125 * | 31.3 | 62.5 * | 31.3 | 125 ** |
| C2 | 15.6 | 62.5 * | 3.9 | 31.3 * | 15.6 | 62.5 ** |
| C3 | 15.6 | 62.5 * | 31.3 | 62.5 * | 7.8 | 31.3 ** |
| C4 | 62.5 | 250 * | 62.5 | 250 * | / | / |
| C5 | 31.3 | 125 * | 31.3 | 125 * | / | / |
| C6 | 125 | >250 * | 125 | >250 * | / | / |
| C7 | 125 | >250 * | 62.5 | 250 * | / | / |
| C8 | 62.5 | 250 * | 62.5 | 125 * | / | / |
| C1:GBP (1:1, wt/wt) | 7.8 Δ | 15.6 Δ | 15.6 Δ | |||
| C2:GBP (1:1, wt/wt) | 15.6 Δ | 3.9 Δ | 15.6 Δ | |||
| C3:GBP (1:1, wt/wt) | 15.6 Δ | 31.3 Δ | 15.6 Δ | |||
| C4:GBP (1:1, wt/wt) | 31.3 Δ | 31.3 Δ | 62.5 Δ | |||
| C5:GBP (1:1, wt/wt) | 15.6 Δ | 15.6 Δ | 31.3 Δ | |||
| C6:GBP (1:1, wt/wt) | 62.5 Δ | 62.5 Δ | 62.5 Δ | |||
| C7:GBP (1:1, wt/wt) | 62.5 Δ | 62.5 Δ | 62.5 Δ | |||
| C8:GBP (1:1, wt/wt) | 62.5 Δ | 62.5 Δ | 62.5 Δ | |||
2.3. Synergistic Antibacterial and Antifungal Effects on GBP with Separated Compounds
| Samples | FIC index | ||
|---|---|---|---|
| S. enterica | S. aureus | A. niger | |
| C1:GBP (1:1, wt/wt) | 0.25 * | 0.5 * | 0.5 * |
| C2:GBP (1:1, wt/wt) | 0.75 Δ | 0.56 Δ | 0.75 Δ |
| C3:GBP (1:1, wt/wt) | 0.75 Δ | 1 Δ | 1.25 ▲ |
| C4:GBP (1:1, wt/wt) | 0.75 Δ | 0.75 Δ | 1 Δ |
| C5:GBP (1:1, wt/wt) | 0.5 * | 0.5 * | 0.5 * |
| C6:GBP (1:1, wt/wt) | 1.25 ▲ | 1.25 ▲ | 1Δ |
| C7:GBP (1:1, wt/wt) | 1.25 ▲ | 1.5 ▲ | 1Δ |
| C8:GBP (1:1, wt/wt) | 1.5 ▲ | 1.5 ▲ | 1Δ |
2.4. Optimal Proportioning Design of Synergistic Effect on GBP with Isophytol against Salmonella Enterica
| Std. Δ | Run | ComponentA: Isophytol (%) ▲ | ComponentB: GBP (%) ▲ | Response1: FIC index | Response2: Diameter of the inhibition halos (mm) |
|---|---|---|---|---|---|
| 10 | 1 | 95.00 | 5.00 | 1 | 10.1 |
| 9 | 2 | 95.00 | 5.00 | 1 | 9.9 |
| 4 | 3 | 27.50 | 72.50 | 0.37 | 15.8 |
| 13 | 4 | 5.00 | 95.00 | 1 | 13.1 |
| 8 | 5 | 5.00 | 95.00 | 1 | 13.0 |
| 5 | 6 | 72.50 | 27.50 | 0.5 | 13.2 |
| 12 | 7 | 95.00 | 5.00 | 1 | 10.1 |
| 3 | 8 | 50.00 | 50.00 | 0.25 | 16.2 |
| 2 | 9 | 95.00 | 5.00 | 1 | 10.2 |
| 7 | 10 | 65.00 | 35.00 | 0.37 | 14.6 |
| 11 | 11 | 5.00 | 95.00 | 1 | 12.9 |
| 1 | 12 | 5.00 | 95.00 | 1 | 13.0 |
| 6 | 13 | 35.00 | 65.00 | 0.25 | 16.3 |


3. Experimental
3.1. Materials
3.2. Extraction and Isolation
3.3. HPLC Analysis
3.4. Determination of Antibacterial and Antifungal Activity
3.5. Determination of Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), Minimum Fungicidal Concentration (MFC), FIC (Fractional Inhibitory Concentration) Index and Determination of the Type of Interactions of Antibacterial and Antifungal Activity
3.6. Optimal Proportioning Design of Synergistic Effect on GBP with Isophytol against Salmonella Enterica
4. Conclusions
Acknowledgments
- Samples Availability: Samples of the compounds: isophytol, nerolidol, linalool,β-sitosterol, stigmasterol, ergosterol and GBP are available from the authors.
References
- van Beek, T.A.; Montorob, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceutical. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef]
- Braquet, P. The Ginkgolides from Chinese Pharmacopeia to a New Class of Pharmacological Agents: The Antagonists of Platelet Activating Factor. In Ginkgolides-Chemistry, Biology, Pharmacologyand Chemical Perspectives, 2nd; Braquet, P., Ed.; Prous Science: Barcelona, Spain, 1988; Volume 1, pp. 15–34. [Google Scholar]
- Ageta, H. Waxes from leaves of Ginkgo biloba and Ephedra gerardiana. J. Pharm. Soc. Jpn. 1959, 79, 58–60. [Google Scholar]
- Kircher, H.W. β-sitosterol in Ginkgo biloba leaves. Phytochemistry 1970, 9, 1879. [Google Scholar] [CrossRef]
- Nguyen Tu, T.T.; Derenne, S.; Largeau, C.; Mariotti, A.; Bocherens, H. Evolution of the chemical composition of Ginkgo biloba external and internal leaf lipids through senescence and litter formation. Org. Geochem. 2001, 32, 45–55. [Google Scholar]
- Wang, C.Z.; Ye, J.Z.; Zheng, G.Y.; Zhang, Z.J.; Shen, Z.B. Studies on separation of sterols from Ginkgo biloba L. by molecular distillation and recrystallization. Chem. Indus. Forest. Prod. 2008, 28, 43–47. [Google Scholar]
- Yang, L.; Wang, C.Z.; Ye, J.Z.; Li, H.T. Hepatoprotective effects of polyprenols from GBL on CCl4-induced hepatotoxicity in rats. Fitoterapia 2011, 82, 834–840. [Google Scholar] [CrossRef]
- Rezanka, T.; Votruba, J. Chromatography of long chain alcohols (polyprenols) from animal and plant sources. J. Chromatogr. A 2001, 936, 95–110. [Google Scholar] [CrossRef]
- Wang, C.Z.; Wang, W.L.; Ye, J.Z.; Zhen, G.Y.; Zhou, H.; Cheng, X.J. Studies on separation of polyprenols from leaves of Ginkgo biloba L. by molecular short distillation. Chem. Indus. For. Prod. 2008, 28, 23–27. [Google Scholar]
- Tao, R.; Wang, C.Z.; Kong, Z.W. Analysis of light distillates isolated by molecular distillation from fat-soluble unsaponifiable matter of Ginkgo biloba leaves based on Py-GC-MS. Chem. Indus. For. Prod. 2012, 32, 66–70. [Google Scholar]
- Bombardelli, E.; Cristoni, A.; Morazzoni, P. Cosmetical Uses of Ginkgo Extracts and Constituents. In Ginkgo biloba, 2nd; van Beek, T.A., Ed.; Harwood Academic Publishers: Singapore, 2000; pp. 475–489. [Google Scholar]
- Sati, S.C.; Joshi, S. Antibacterial activities of Ginkgo biloba L. leaf extracts.Sci. World J. 2011, 11, 2237–2242. [Google Scholar] [CrossRef]
- Choi, J.G.; Jeong, S.I.; Ku, C.S.; Sathishkumar, M.; Lee, J.J.; Mun, S.P.; Kim, S.M. Antibacterial activity of hydroxyalkenyl salicylic acids from sarcotesta of Ginkgo biloba against vancomycin-resistant Enterococcus. Fitoterapia 2009, 80, 18–20. [Google Scholar] [CrossRef]
- Xu, J.H.; Lu, Q.H.; Zhao, Y. Studies on chemical constituents of green algae Ulva pertusa. Chin. J. Mater. Med. 2007, 32, 1536–1538. [Google Scholar]
- Miyazawa, M.; Nankai, H.; Kameoka, H. Biotransformations of acyclic terpenoids, (±)-trans-nerolidol and geranylacetone, by Glomerella cingulata. J. Agric. Food Chem. 1996, 44, 1543–1547. [Google Scholar] [CrossRef]
- Sun, W.J. The Concise Handbook of Natural Bioactive Constituents, 2nd; Sun, W.J., Ed.; Chinese Medicinal Science and Technology Press: Beijing, China, 1998; p. 350. [Google Scholar]
- Zhan, Q.; Wang, Y.; Li, X.; Chen, W.S.; Sun, L.N. Studies on the chemical constituents of petroleum ether extract of Lagerstroemia speciosa (Linn.) Pers leaves. Lishizhen Med. Mat. Med. Res. 2009, 20, 2125–2127. [Google Scholar]
- Kojim, H.; Sato, N.; Hatanoa, A.; Ogura, H. Sterol glucosides from Prunella vulgaris. Phytochemistry 1990, 29, 2351–2355. [Google Scholar] [CrossRef]
- Huang, L.; Cao, Y.; Xu, H.; Chen, G. Separation and purification of ergosterol and stigmasterol in Anoectochilus roxburghii (wall) Lindl by high-speed counter-current chromatography. J. Sep. Sci. 2011, 34, 385–392. [Google Scholar] [CrossRef]
- Hyun, S.K.; Jung, H.A.; Chung, H.Y.; Choi, J.S. In vitro peroxynitrite scavenging activity of 6-hydroxykynurenic acid and other flavonoids from Gingko biloba yellow leaves. Arch. Pharm. Res. 2006, 29, 1074–1079. [Google Scholar] [CrossRef]
- Karolina, T.C.; Skorupinska-Tudek, K.; Hertel, J.; Chojnacki, T.; Olsson, J.M.; Swiezewska, E. Single polyprenol and dolichol isolation by semipreparative high-performance liquid chromatography technique. J. Lipid Res. 2000, 41, 1177–1180. [Google Scholar]
- Singh, B.; Kaur, P.; Gopichand; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef]
- van Beek, T.A. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–55. [Google Scholar] [CrossRef]
- Park, M.J.; Gwak, K.S.; Yang, I.; Kim, K.W.; Jeung, E.B.; Chang, J.W.; Choia, I.G. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia 2009, 80, 290–296. [Google Scholar] [CrossRef]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, Farnesol, Bisabolol, and apritone. Antimicrob. Agents Ch. 2003, 47, 3357–3360. [Google Scholar] [CrossRef]
- Wang, C.Z.; Shen, Z.B. The effects on transplanted Heps and EC tumors in mouse for polyprenols in combination with chemotherapeutic drugs. Chin. J. Biochem. Pharm. 2003, 24, 113–115. [Google Scholar]
- Wang, C.Z.; Shen, Z.B.; Gao, L. Pharmacology of polyprenols from Ginkgo biloba L. leaves in combination with chemotherapeutic drugs on tumor. Chem. Indus. For. Prod. 2003, 23, 15–18. [Google Scholar]
- Wang, C.Z.; Shen, Z.B.; Tan, W.H.; Yu, Q.; Chen, X. Preparation and utilization of polyprenols and extractive from GBL. Chinese Patent ZL01113696.0, 2004. [Google Scholar]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar]
- Bougatsos, C.; Meyer, J.J.M.; Magiatis, P.; Vagias, C.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of Helichrysum kraussii Sch. Bip. and H. rugulosum Less. from south Africa. Flavour Fragr. J. 2003, 18, 48–51. [Google Scholar] [CrossRef]
- Senatore, F.; Napolitano, F.; Arnold, N.A.; Bruno, M.; Herz, W. Composition and antimicrobial activity of the essential oil of Achillea falcata L. (Asteraceae). Flavour Fragr. J. 2005, 2, 291–294. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar]
- Hall, M.J.; Middleton, R.F.; Westmacott, D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 1983, 11, 427–433. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebié, R.C.; Dicko, M.H. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tao, R.; Wang, C.-Z.; Kong, Z.-W. Antibacterial/Antifungal Activity and Synergistic Interactions between Polyprenols and Other Lipids Isolated from Ginkgo Biloba L. Leaves. Molecules 2013, 18, 2166-2182. https://doi.org/10.3390/molecules18022166
Tao R, Wang C-Z, Kong Z-W. Antibacterial/Antifungal Activity and Synergistic Interactions between Polyprenols and Other Lipids Isolated from Ginkgo Biloba L. Leaves. Molecules. 2013; 18(2):2166-2182. https://doi.org/10.3390/molecules18022166
Chicago/Turabian StyleTao, Ran, Cheng-Zhang Wang, and Zhen-Wu Kong. 2013. "Antibacterial/Antifungal Activity and Synergistic Interactions between Polyprenols and Other Lipids Isolated from Ginkgo Biloba L. Leaves" Molecules 18, no. 2: 2166-2182. https://doi.org/10.3390/molecules18022166
APA StyleTao, R., Wang, C. -Z., & Kong, Z. -W. (2013). Antibacterial/Antifungal Activity and Synergistic Interactions between Polyprenols and Other Lipids Isolated from Ginkgo Biloba L. Leaves. Molecules, 18(2), 2166-2182. https://doi.org/10.3390/molecules18022166