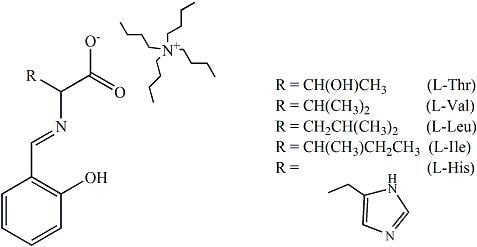
Spectroscopic Studies of Amino Acid Ionic Liquid-Supported Schiff Bases
Abstract
:1. Introduction

2. Results and Discussion
2.1. NMR, IR and UV-Vis

| Comp. | T (K) | Position | 3J(NH.H) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | α | 2' | 1' | Others | |||||
| (1) Sal-L-Thr | 295 | CDCl3 | δH (ppm) | - | 14.41 | 6.85 | ~7.25 | 6.78 | ~7.25 | 8.45 | - | 4.16 | 3.92;3.18;1.56;1.37;1.20;0.95 | n.o. |
| δC (ppm) | 119.01 | 165.20 | 117.46 | 132.06 | 117.59 | 131.50 | 163.14 | 173.97 | 76.09 | 68.13;58.54;23.86;19.67;19.11;13.65 | ||||
| ΔC(D) (ppb) | n.o. | 136 | n.o. | n.o. | n.o. | n.o. | 451 | n.o. | −174 | |||||
| 270 | CDCl3 | δH (ppm) | - | 14.49 | 6.85 | ~7.3 | 6.80 | ~7.3 | 8.46 | - | 4.17 | 3.94;3.16;1.54;1.36;1.21;0.95 | n.o. | |
| δC (ppm) | 118.81 | 165.27 | 117.36 | 132.12 | 117.69 | 131.48 | 162.85 | 174.07 | 75.93 | 68.00;58.23;23.67;19.59;19.05;13.69 | ||||
| ΔC(D) (ppb) | −155 | 174 | -88 | 57 | 152 | −72 | 527 | 77 | −178 | |||||
| 250 | CDCl3 | δH (ppm) | - | 14.55 | 6.87 | ~7.3 | 6.83 | ~7.3 | 8.46 | - | 4.18 | 3.96;3.16;1.53;1.34;1.23;0.96 | n.o. | |
| δC (ppm) | 118.65 | 165.36 | 117.30 | 132.20 | 117.79 | 131.49 | 162.62 | 174.18 | 75.78 | 67.91;58.00;23.52;19.54;19.01;13.75 | ||||
| ΔC(D) (ppb) | −145 | 174 | −86 | 67 | 139 | −64 | 535 | 74 | −178 | |||||
| 230 | CDCl3 | δH (ppm) | - | 14.62 | 6.89 | 7.36 | 6.85 | 7.31 | 8.47 | - | 4.18 | 3.99;3.14;1.53;1.33;1.23;0.96 | n.o. | |
| δC (ppm) | 118.48 | 165.41 | 117.26 | 132.30 | 117.88 | 131.49 | 162.41 | 174.28 | 75.57 | 67.81;57.77;23.38;19.50;18.97;13.81 | ||||
| ΔC(D) (ppb) | 136 | 174 | −86 | 68 | 149 | −73 | 540 | 83 | −235 | |||||
| 295 | DMSO | δH (ppm) | - | 14.32 | 6.76 | 7.24 | 6.74 | 7.34 | 8.37 | - | 3.81 | 3.60;3.15;1.55;1.30;1.02;0.92 | n.o. | |
| δC (ppm) | 118.26 | 164.49 | 116.65 | 132.06 | 117.40 | 131.51 | 163.68 | 170.95 | 75.10 | 67.35;57.38;22.95;19.44;19.11;13.40 | ||||
| (2)Sal-L-Val a | 295 | CDCl3 | δH (ppm) | - | 14.75 | 6.79 | 7.23 | 6.66 | 7.17 | 8.21 | - | 3.71 | 3.24;1.57;1.35;1.00;0.94 | n.o. |
| δC (ppm) | 117.85 | 167.22 | 119.11 | 132.89 | 115.95 | 131.85 | 163.39 | 173.44 | 79.11 | 58.57;31.45;23.94;19.70;18.34;13.67 | ||||
| ΔC(D) (ppb) | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | |||||
| 270b | CDCl3 | δH (ppm) | - | 14.90 | 6.79 | 7.24 | 6.65 | 7.18 | 8.17 | - | 3.73 | 3.24;1.57;1.35;0.99;0.94 | br. | |
| δC (ppm) | 117.38 | 168.07 | 119.46 | 133.21 | 115.61 | 131.93 | 163.34 | 173.28 | 78.58 | 58.28;31.32;23.77;19.62;18.14;13.72 | ||||
| ΔC(D) (ppb) | n.o. | 74 c | n.o. | −48 | −51 | −135 | 269 | n.o. | n.o. | |||||
| 250 | CDCl3 | δH (ppm) | - | 14.89 | 6.80 | 7.28 | 6.67 | 7.20 | 8.16 | - | 3.76 | 3.20;1.55;1.33;1.00;0.93 | 6.1 | |
| δC (ppm) | 117.07 | 168.38 | 119.58 | 133.46 | 115.55 | 132.04 | 163.51 | 173.43 | 78.06 | 58.00;31.21;23.58;19.56;18.45;17.65;13.83 | ||||
| ΔC(D) (ppb) | 29 | −73 | n.o. | −75 | −105 | −156 | 271 | n.o. | n.o. | |||||
| 230 | CDCl3 | δH (ppm) | - | 15.00 | 6.80 | 7.31 | 6.67 | 7.22 | 8.14 | - | 3.81 | 3.20;1.54;1.31;1.00;0.94 | 7.1 | |
| δC (ppm) | 116.61 | 169.66 | 120.09 | 133.91 | 115.19 | 132.18 | 163.52 | 173.35 | ~77.4 | 57.78;31.09;23.43;19.52;18.45;17.65;13.83 | ||||
| ΔC(D) (ppb) | 80 | br. | br. | −183 | −149 | −118 | 283 | n.o. | n.o. | |||||
| 295 | DMSO | δH (ppm) | - | 14.54 | 6.68 | 7.29 | 6.62 | 7.21 | 8.28 | - | 3.44 | 3.15;2.26;1.55;1.29;1.04;0.92;0.84;0.82 | n.o. | |
| δC (ppm) | 117.68 | 166.36 | 118.49 | 132.56 | 115.55 | 131.85 | 163.35 | 170.63 | 77.98 | 57.50;56.01;30.86;23.08;20.27;19.23;18.58;18.11;13.52 | ||||
| (3)Sal-L-Leu | 295 | CDCl3 | δH (ppm) | - | 14.76 | 6.79 | 7.22 | 6.68 | 7.18 | 8.32 | - | 4.02 | 3.23;1.91;1.68;1.57;1.37;0.96;0.92;0.89 | n.o. |
| δC (ppm) | 118.20 | 166.11 | 118.73 | 132.60 | 116.34 | 131.84 | 163.14 | 174.21 | 71.25 | 58.59;43.09;25.03;23.93;21.50;19.71;18.49;13.67 | ||||
| ΔC(D) (ppb) | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | |||||
| 270 | CDCl3 | δH (ppm) | - | 14.83 | 6.80 | 7.25 | 6.69 | 7.20 | 8.30 | - | 4.03 | 3.20;1.91;1.63;1.56;1.35;0.95;0.91;0.88 | n.o. | |
| δC (ppm) | 117.83 | 166.36 | 118.85 | 132.83 | 116.25 | 131.90 | 163.22 | 174.30 | 70.85 | |||||
| ΔC(D) (ppb) | n.o. | 248 | n.o. | n.o. | -47 | −103 | 248 | n.o. | -113 | 58.24;42.77;24.83;23.71;21.18;19.63;18.50;13.73 | ||||
| 250 | CDCl3 | δH (ppm) | - | 14.84 | 6.81 | ~7.3 | 6.71 | 7.22 | 8.30 | - | 4.05 | 3.17;1.92;1.54;1.32;0.95;0.88 | 3.3 | |
| δC (ppm) | 117.20 | 166.87 | 119.00 | 133.04 | 116.10 | 131.96 | 163.56 | 174.37 | 70.35 | 58.00;42.51;24.66;23.71;20.92;19.57;18.46;13.77 | ||||
| ΔC(D) (ppb) | n.o. | 198 | n.o. | n.o. | −44 | −121 | 259 | n.o. | -104 | |||||
| 230 | CDCl3 | δH (ppm) | - | 14.95 | 6.82 | 7.30 | 6.72 | 7.24 | 8.27 | - | 4.06 | 3.18;1.94;1.54;1.33;0.95;0.91;0.87 | 7.3 | |
| δC (ppm) | 117.19 | 167.24 | 119.23 | 133.31 | 116.03 | 131.93 | 163.68 | 174.45 | 69.88 | 57.78;42.24;24.51;23.76;20.64;19.53;13.84 | ||||
| ΔC(D) (ppb) | n.o. | 146 | n.o. | n.o. | n.o. | 869 | 256 | n.o. | -76 | |||||
| 350 | DMSO | δH (ppm) | - | 14.20 | 6.69 | 7.19 | 6.64 | 7.26 | 8.35 | - | 3.72 | 3.20;1.78;1.61;1.34;0.94;0.88;0.86 | n.o. | |
| δC (ppm) | 118.59 | 166.95 | 118.64 | 132.52 | 116.12 | 132.0 | 162.95 | 171.26 | 71.13 | 58.53;43.89;25.37;23.75;22.44;19.69;13.73 | ||||
| 330 | δH (ppm) | - | 14.4 | 6.69 | 7.20 | 6.64 | 7.28 | 8.35 | - | 3.70 | 3.19;1.75;1.61;1.32;0.93;0.88;0.86 | |||
| δC (ppm) | 118.51 | 166.14 | 118.62 | 132.61 | 116.17 | 132.06 | 163.07 | 171.39 | 71.10 | 58.31;43.79;25.31;23.74;22.35;19.68;13.82 | ||||
| 295 | δH (ppm) | - | 14.53 | 6.68 | 7.28 | 6.63 | 7.20 | 8.33 | - | 3.66 | 3.16;1.90;1.55;1.29;0.92;0.86;0.83 | |||
| δC (ppm) | 117.70 | 165.73 | 118.15 | 132.25 | 115.59 | 131.63 | 162.69 | 170.81 | 70.33 | 57.37;43.01;24.61;23.28;22.95;21.55;19.10;13.39 | ||||
| (4)Sal-L-Ile | 295 | CDCl3 | δH (ppm) | - | 14.81 | 6.79 | 7.23 | 6.64 | 7.16 | 8.18 | - | 3.74 | 3.23;2.87;2.24;1.66;1.56;1.35;1.23;0.97;0.93;0.88 | n.o. |
| δC (ppm) | 117.77 | 167.56 | 119.31 | 133.01 | 115.86 | 131.90 | 163.18 | 173.56 | 78.74 | 58.60;37.95;24.98;23.95;19.72;16.51;13.67;11.56 | ||||
| ΔC(D) (ppb) | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | |||||
| 270 | CDCl3 | δH (ppm) | - | 14.89 | 6.78 | 7.24 | 6.65 | 7.18 | 8.16 | - | 3.78 | 3.23;2.27;1.68;1.56;1.33;1.23;1.16;0.96;0.91;0.88 | br. | |
| δC (ppm) | 117.31 | 168.24 | 119.55 | 133.30 | 115.60 | 131.96 | 163.24 | 173.52 | 78.28 | 58.24;37.75;24.69;23.73;19.63;16.44;13.72;11.61 | ||||
| ΔC(D) (ppb) | n.o. | −43 c | −58 | 35 | n.o. | −145 | 254 | n.o. | n.o. | |||||
| 250 | CDCl3 | δH (ppm) | - | 14.94 | 6.79 | 7.27 | 6.66 | 7.19 | 8.14 | - | 3.80 | 3.21;2.28;1.68;1.56;1.30;1.23;1.16;0.95;0.90;0.88 | 6.0 | |
| δC (ppm) | 116.94 | 168.78 | 119.78 | 133.60 | 115.42 | 132.05 | 163.30 | 173.57 | 77.87 | 58.01;37.63;24.44;23.57;19.57;16.38;13.77;11.67 | ||||
| ΔC(D) (ppb) | 59 | −41 | −112 | -83 | n.o. | −164 | 276 | n.o. | 101 | |||||
| 230 | CDCl3 | δH (ppm) | - | 15.03 | 6.79 | 7.31 | 6.64 | 7.21 | 8.10 | - | 3.85 | 3.22;2.33;1.70;1.55;1.32;1.23;1.17;0.95;0.90;0.88 | 7.2 | |
| δC (ppm) | 116.46 | 169.85 | 120.24 | 134.02 | 115.03 | 132.22 | 163.37 | 173.46 | 77.41 | 57.78;37.49;24.15;23.43;19.54;16.35;13.83;11.76 | ||||
| ΔC(D) (ppb) | 85 | -143 | −150 | −134 | 95 | −158 | 295 | n.o. | ov. | |||||
| 295 | DMSO | δH (ppm) | - | 14.57 | 6.66 | 7.27 | 6.60 | 7.20 | 8.26 | - | 3.44 | 3.15;1.99;1.50;1.29;1.00;0.93;0.83 | ||
| δC (ppm) | 117.56 | 166.83 | 118.67 | 132.58 | 115.27 | 131.86 | 163.05 | 170.24 | 77.37 | 57.49;37.56;24.63;23.06;19.22;16.60;13.52;11.67 | ||||
| (5) Sal-L-His | 295 | CDCl3 | δH (ppm) | - | 12.33 | 6.63 | ~7.3 | 6.80 | ~7.3 | 7.53 | - | 5.19 | 3.75;3.58;3.32;3.14;2.94;1.54;1.30;0.92 | |
| δC (ppm) | ~123.7 | ~157.3 | 116.22 | ~128.3 | 118.88 | ~129.6 | n.o. | n.o. | 58.67 | 58.31;~26.45;23.93;19.69;13.67 | ||||
| ΔC(D) (ppb) | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | |||||
| 270 | CDCl3 | δH (ppm) | - | 12.75 | 6.63 | 7.08 | 6.81 | ~7.3 | 7.47 | - | 5.20 | 11.82;3.77;3.36;3.09;2.98;1.52;1.26;0.91 | ||
| δC (ppm) | 123.71 | 157.11 | 116.10 | 126.47 | 118.88 | 129.46 | 135.53 | 175.35 | 59.81 | 58.31;27.34;23.70;19.57;13.69 | ||||
| ΔC(D) (ppb) | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. | |||||
| 250 | CDCl3 | δH (ppm) | - | 12.91 | 6.64 | 7.09 | 6.83 | ~7.3 | 7.44 | - | 5.19 | 11.87;3.80;3.65;3.40;3.07;~3.0;1.51;1.25;0.91 | ||
| δC (ppm) | 123.74 | 156.87 | 116.05 | 126.21 | 118.89 | 129.47 | 135.10 | 175.60 | 59.64 | 134.87;58.05;27.15;23.55;19.49;13.72 | ||||
| ΔC(D) (ppb) | 157 | n.o. | n.o. | n.o. | n.o. | 136 a | 393 | n.o. | -88 a | |||||
| 230 | CDCl3 | δH (ppm) | - | 13.06 | 6.65 | 7.11 | 6.85 | ~7.3 | 7.47 | - | 5.20 | 11.91;3.83;3.67;3.43;3.05;1.51;1.25;0.91 | ||
| δC (ppm) | 123.58 | 156.82 | 115.98 | 126.16 | 118.88 | 129.38 | 135.01 | 175.69 | 59.49 | 128.24;57.26;26.94;23.44;19.45;13.77 | ||||
| ΔC(D) (ppb) | 143 | br. | -41 c | -28 c | 64 | br. | br. | n.o. | br. | 40 | ||||
| 295 | DMSO | δH (ppm) | - | 12.1 | 6.61 | 7.06 | 6.74 | 7.19 | 7.30 | - | 5.04 | ~11.6;3.19;3.14;~2.9;1.57;1.33;0.96 | ||
| δC (ppm) | 124.47 | 157.79 | 116.41 | 127.80 | 118.38 | 129.85 | 133.85 | 172.83 | 58.91 | 136.09;129.85;58.0;26.72;23.57;19.72; 14.00 | ||||


| Comp. | v(XH) | v(C=N) | v(COO−)as. | v(COO−)sym. |
|---|---|---|---|---|
| (1) S-Thr | 3700–2700 br. | 1630 | 1613 | 1377 |
| (2) S-Val | 3750–2700 br. | 1630 | 1609 | 1368 |
| (3) S-Leu | 3700–2700 br. | 1629 | 1606 | 1361 |
| (4) S-Ile | 3700–2875 br. | 1629 | 1608 | 1361 |
| (5) S-His | 3500–2400 br. | 1597 | 1597 ov. | 1391 |
| Comp. | EtOH | CHCl3 | ||
|---|---|---|---|---|
| (1) Sal-L-Thr | 288 (0.399) | 404 (0.278) | 292 (0.152) | 419 (0.038) |
| 317 (0.447) | 315 (0.138) | |||
| (2) Sal-L-Val | 289 (0.718) | 404 (0.584) | 288 (0.273) | 408 (0.188) |
| 316 (0.699) | 315 (0.2) | |||
| (3) Sal-L-Leu | 289 (0.596) | 405 (0.429) | 291 (0.478) | 410 (0.315) |
| 315 (0.589) | 315 (0.404) | |||
| (4) Sal-L-Ile | 288 (0.627) | 407 (0.536) | 289 (0.27) | 408 (0.175) |
| 315 (0.572) | 313 (0.185) | |||
| (5) Sal-L-His | 290 (1.218) | 407 (0.137) | 289 (0.926) | n.o. |
| Comp. | [α]λT | [M]λT |
|---|---|---|
| (1) Sal-L-Thr | +13.6 | +63.2 |
| (2) Sal-L-Val | +29.7 | +137.4 |
| (3) Sal-L-Leu | +4.9 | +23.4 |
| (4) Sal-L-Ile | +17.7 | +84.4 |
| (5) Sal-L-His | +0.7 | +50.1 |
2.2. ES MS


3. Experimental
General

4. Conclusions
Supplementary Materials
Acknowledgments
References
- Stark, A.; Seddon, K.R. Ionic Liquids. In Chemical Technology and the Environment, 1st; Kirk-Othmer, Ed.; Wiley-Interscience: New York, NY, USA, 2007; Volume 1, pp. 308–392. [Google Scholar]
- Earle, M.; Seddon, K. Ionic liquids. Green solvent for the future. Pure App. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Dramska, A.; Antoszczyszyn, M.; Janus, E. Reakcja Dielsa i Aldera w cieczach jonowych z dodatkiem trifluorometanosulfonianów skandu i litu jako katalizatorów. Przem. Chem. 2006, 85, 47–49. [Google Scholar]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, L.; Ye, Y.; Chen, L.; Qi, Z.; Freund, H.; Sundmacher, K. An Overview of Mutual Solubility of Ionic Liquids and Water Predicted by COSMO-RS. Ind. Eng. Chem. Res. 2012, 51, 6256–6264. [Google Scholar]
- Keskin, S.; Kayrak-Talay, D.; Akman, U.; Hortaçsu, Ö. A review of ionic liquids towards supercritical fluid applications. J. Supercrit. Fluids 2007, 43, 150–180. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Ionic Liquids as Green Solvents: Properties and Prospects. In Green Solvents II—Properties and Applications of Ionic Liquids, 1st; Mohammad, A., Inamuddin, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; Chapter 1; pp. 1–32. [Google Scholar]
- Domínguez de Maria, P. Ionic Liquids: Deffinitions, Applications, and Context for Biotransformations and Organocatalysis. In Ionic Liquids in Biotransformation and Organocatalysis: Solvents and Beyond, 1st; Domínguez de Maria, P., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2012; Chapter 1; pp. 1–14. [Google Scholar]
- Hu, S.; Jiang, T.; Zhang, Z.; Zhu, A.; Han, B.; Song, J.; Xie, Y.; Li, W. Functional ionic liquid from biorenewable materials: Synthesis and application as a catalyst in direct aldol reactions. Tetrahedron Lett. 2007, 48, 5613–5617. [Google Scholar] [CrossRef]
- Payagala, T.; Armstrong, D.W. Chiral Ionic Liquids: A Compendium of Syntheses and Applications (2005–2012). Chirality 2012, 24, 17–53. [Google Scholar] [CrossRef]
- Khungar, B.; Rao, M.S.; Pericherla, K.; Nehra, P.; Jain, N.; Panwar, J.; Kumar, A. Synthesis, characterization and microbiocidal studies of novel ionic liquidtagged Schiff bases. C. R. Chimie 2012, 15, 669–674. [Google Scholar]
- Ouadi, A.; Gadenne, B.; Hesemann, P.; Moreau, J.J.E.; Billard, I.; Gaillard, C.; Soufiane, M.; Moutiers, G. Task-Specific Ionic Liquids Bearing 2-Hydroxybenzylamine Units: Synthesis and Americium-Extraction Studies. Chem. Eur. J. 2006, 12, 3074–3081. [Google Scholar] [CrossRef]
- Chan-Hout, M.; Sharif, S.; Tolstoy, P.M.; Toney, M.D.; Limbach, H.H. NMR Studies of Stability, Protonation Site, and Tautomerism of 13C- and 15N-Labeled Aldimines of the Coenzyme Pyridoxal 5'-Phosphate in Water. Biochemistry 2010, 49, 10818–18830. [Google Scholar] [CrossRef]
- Christen, P.; Metzler, D.E. Transaminases, 1st ed; Wiley & Sons: New York, NY, USA, 1985; pp. 37–101. [Google Scholar]
- Li, B.; Li, Y.-Q.; Zheng, W.-J.; Zhou, M.-Y. Synthesis of ionic liquid-supported Schiff bases. Arkivoc 2009, XI, 165–171. [Google Scholar]
- Li, B.; Zheng, Y.-Q.; Li, J. A novel ionic liquid-supported Schiff base ligand applied in the Pd-catalyzed Suzuki-Miyaura coupling reaction. Arkivoc 2010, IX, 163–170. [Google Scholar]
- Ohlin, C.A.; Dyson, P.J.; Laurenczy, G. Carbon monoxide solubility in ionic liquids: determination, prediction and relevance to hydroformylation. Chem. Commun. 2004, 2004, 1070–1071. [Google Scholar]
- Vidiš, A.; Ohlin, C.A.; Laurenczy, G.; Kűsters, E.; Sedelmeier, G.; Dyson, P.J. Rationalisation of Solvent Effects in the Diels-Alder Reaction Between Cyclopentadiene and Methyl Acrylate in Room Temperature Ionic Liquids. Adv. Synth. Catal. 2005, 347, 266–274. [Google Scholar] [CrossRef]
- Rozwadowski, Z. Deuterium isotope effect on 13C chemical Shift of tetrabutylammonium salts of Schiff bases amino acids. Magn. Reson. Chem. 2006, 44, 881–886. [Google Scholar] [CrossRef]
- Rozwadowski, Z.; Ambroziak, K.; Szypa, M.; Jagodzińska, E.; Spychaj, S.; Schilf, W.; Kamieński, B. The 15N and 13C-NMR study of Schiff bases derivatives of amino acids and their lithium salts in solid state and DMSO. J. Mol. Struct. 2005, 734, 137–142. [Google Scholar] [CrossRef]
- Rozwadowski, Z. Deuterium isotope effects on 13C chemical shifts of lithium salts of Schiff bases amino acids. J. Mol. Struct. 2006, 753, 127–131. [Google Scholar] [CrossRef]
- Dziembowska, T.; Hansen, P.E.; Rozwadowski, Z. Studies based on deuterium isotope effect on 13C chemical shifts. Prog. Nucl. Magn. Reson. Spectrosc. 2004, 45, 1–29. [Google Scholar] [CrossRef]
- Rozwadowski, Z. NMR Studies of optically active Schiff bases. Ann. Rep. NMR Spectrosc. 2011, 74, 125–180. [Google Scholar]
- Rozwadowski, Z.; Majewski, E.; Dziembowska, T.; Hansen, P.E. Deuterium isotope effects on 13C chemical shifts of intramolecularly hydrogen-bonded Schiff bases. J. Chem. Soc. Perkin Trans. 2 1999, 1999, 2809–2817. [Google Scholar]
- Özcan, Y.; Ide, S.; Şakiyan, I.; Logoglu, E. Structure and characterization of N-(2-hydroxy-1-naphthylidene)threonine. J. Mol. Struct. 2003, 658, 207–213. [Google Scholar]
- Przybylski, P.; Pyta, K.; Ratajczak-Sitarz, M.; Katrusiak, A.; Brzezinski, B. X-ray, FT-IR, ESI MS and PM5 studies of Schiff base of gossypol with allylamine and its complexes with alkali metal cations and perchlorate anion. Struct. Chem. 2008, 19, 983–995. [Google Scholar] [CrossRef]
- Rubčić, M.; Užarević, K.; Halasz, I.; Bregović, N.; Mališ, M.; Dilović, I.; Kokan, Z.; Stein, R.S.; Dinnebier, R.E.; Tomišić, V. Desmotropy, Polymorphism, and Solid-State Proton Transfer: Four Solid Forms of an Aromatic o-Hydroxy Schiff Base. Chem. Eur. J. 2012, 18, 5620–5631. [Google Scholar] [CrossRef]
- Gűngör, Ö.; Gűrkan, P. Synthesis and spectroscopic properties of novel asymmetric Schiff bases. Spectrochim. Acta A 2010, 77, 304–311. [Google Scholar] [CrossRef]
- Herzfeld, R.; Nagy, P. Studies of the Solvent Effect Observed in the Absorption Spectra of Certain Types of Schiff Bases. Curr. Org. Chem. 2001, 5, 373–394. [Google Scholar] [CrossRef]
- Zeng, Q.-L.; Chen, W-Z.; Zhao, Y.-F. Electrospray ionization mass spectral fragmentation study of amino acid derived oxovanadium Schiff base complexes and (oxo)-peroxovanadium Schiff base complexes. Inter. J. Mass Spectr. 2007, 262, 161–167. [Google Scholar]
- Gilbert, W.C.; Taylor, L.T.; Dillard, J.G. Mass-spectrometric study of polydentate Schiff-base coordination compounds. I. Cobalt(II), nickel(II), and copper(II) complexes of Salen [bis(salicylidene)ethylenediamine] and Oaben [bis(o-aminobenzylidene) ethylenediamine]. J. Am. Chem. Soc. 1973, 95, 2477–2482. [Google Scholar]
- Dubey, R.K.; Mariya, A.; Mishra, S.K. Synthesis and spectral (ir, nmr, fab-ms and xrd) characterization of lanthanide complexes containing bidentate schiff base derived from sulphadiazine and o-vanillin. Int. J. Basic Appl. Chem. Sci. 2011, 1, 70–78. [Google Scholar]
- Salmann, S.R.; Saleh, N.A.I. Mass Spectral Study of Tautomerism in Some Schiff Bases. Spectrosc. Lett. 1998, 31, 1179–1189. [Google Scholar] [CrossRef]
- Allen, C.R.; Richard, P.L.; Ward, A.J.; van de Water, L.G.A.; Masters, A.F.; Maschmeyer, T. Facile synthesis of ionic liquids possessing chiral carboxylates. Tetrahedron Lett. 2006, 47, 7367–7370. [Google Scholar]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ossowicz, P.; Janus, E.; Schroeder, G.; Rozwadowski, Z. Spectroscopic Studies of Amino Acid Ionic Liquid-Supported Schiff Bases. Molecules 2013, 18, 4986-5004. https://doi.org/10.3390/molecules18054986
Ossowicz P, Janus E, Schroeder G, Rozwadowski Z. Spectroscopic Studies of Amino Acid Ionic Liquid-Supported Schiff Bases. Molecules. 2013; 18(5):4986-5004. https://doi.org/10.3390/molecules18054986
Chicago/Turabian StyleOssowicz, Paula, Ewa Janus, Grzegorz Schroeder, and Zbigniew Rozwadowski. 2013. "Spectroscopic Studies of Amino Acid Ionic Liquid-Supported Schiff Bases" Molecules 18, no. 5: 4986-5004. https://doi.org/10.3390/molecules18054986
APA StyleOssowicz, P., Janus, E., Schroeder, G., & Rozwadowski, Z. (2013). Spectroscopic Studies of Amino Acid Ionic Liquid-Supported Schiff Bases. Molecules, 18(5), 4986-5004. https://doi.org/10.3390/molecules18054986