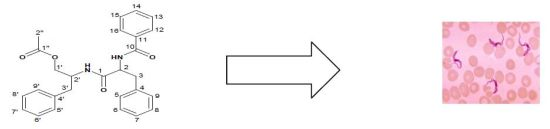
Two Trypanocidal Dipeptides from the Roots of Zapoteca portoricensis (Fabaceae)
Abstract
:1. Introduction
2. Results and Discussion


| Compound | T. b. rhodesiense | T.cruzi | L6 cells |
|---|---|---|---|
| IC50 µM | IC50 µM | IC50 µM | |
| 1 | 3.63 | 41.65 | 92.05 |
| 2 | 12.21 | 16.05 | 71.20 |
| Melarsoprol | 0.003 | - | - |
| Benznidazole | - | 0.407 | - |
| Podophyllotoxin | - | - | 0.008 |
3. Experimental
3.1. General Procedures
3.2. Plant Materials
3.3. Exraction and Isolation
3.4. Determination of in Vitro Antitrypanosomal Activity and Cytotoxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nwodo, N.J.; Uzochukwu, C.I. Studies on anti-inflammatory and antimicrobial activities of crude methanol extracts of Zapoteca portoricensis Jacq. H. Hernanadez. Recent Prog. Med. Plants 2008, 19, 61–69. [Google Scholar]
- Nwodo, N.J.; Omeje, E.O.; Brun, R. In vitro–in vivo studies on anti-trypanosomal potentials of Zapoteca portoricensis. Asian Pac. J. Trop. Med. 2009, 2, 25–29. [Google Scholar]
- Agbo, M.O.; Okoye, F.B.C.; Nwodo, N.J. In vivo anti-inflammatory effect of Zapoteca portoricensis (Jacq) HM Hernández. Inter. J. Health Res. 2010, 3, 29–35. [Google Scholar]
- Esimone, C.O.; Onuh, P.U.; Obitte, N.C.; Egege, M.K.; Ugoeze, K.C. In vitro evaluation of lozenges containing extracts of roots of Zapoteca portoricensis (FAM: Fabaceae). J. Pharmacol. Toxicol. 2009, 4, 132–137. [Google Scholar] [CrossRef]
- Ukwe, C.V.; Ubaka, C.M.; Adibe, M.O.; Okonkwo, C.J.; Akah, P.A. Antiulcer activity of roots of Zapoteca portoricensis (Fam. Fabaceae). J. Basic Clin. Pharm. 2010, 1, 183–186. [Google Scholar]
- Agbafor, K.N.; Akubugwu, E.I.; Ogbashi, N.E.; Ajah, P.M.; Ukwandu, C.C. Chemical and Antimicrobial properties of leaf extracts of Zapoteca portoricensis. Res. J. Med. Plants 2011, 5, 605–612. [Google Scholar] [CrossRef]
- Danquah, M.K.; Agyei, D. Pharmaceutical applications of bioactive peptides. OA Biotechnol. 2012, 1, 5. [Google Scholar]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Ishiguro, K.; Nagata, S.; Fukumoto, H.; Yamaki, M.; Takagi, S.; Isoi, S. A dipeptide derivative from Hypericum japonicum. Phytochemistry 1991, 30, 3639–3641. [Google Scholar] [CrossRef]
- Hashim, N.M.; Rahmani, M.; Shamaun, S.S.; Ee, G.C.L.; Sukari, M.A.; Ali, A.M.; Go, R. Dipeptide and xanthones from Artocarpus kemando Miq. J. Med. Plant Res. 2011, 5, 4224–4230. [Google Scholar]
- Kouam, J.L.; Dongo, E.; Mpondo, T.N.; White, R.L. Chemical constituents from stem bark and roots of Clausena anisata. Molecules 2012, 17, 13673–13686. [Google Scholar] [CrossRef]
- Ferreira, D.T.; Silva, R.B.; Deoliveira, A.B.; Isobe, M.; Braz, R. Dipeptide from the root of Zeyhera digitalis. J. Braz. Chem. Soc. 1995, 6, 323–326. [Google Scholar] [CrossRef]
- Chang, R.; Wang, C.; Zeng, Q.; Guan, B.; Zhang, W.; Jin, H. Chemical constituents of the stems of Celastrus rugosus. Arch. Pharm. Res. 2013, 36, 1291–1301. [Google Scholar] [CrossRef]
- Carvalho, M.G.; Cardozo, M.A.; Catunda, F.E., Jr.; Carvalho, A.G. Chemical constituents of Piptadenia gonoacantha J.F. Macbr. Ann. Braz. Acad. Sci. 2010, 82, 561–567. [Google Scholar]
- Kuo, P.C.; Hwang, T.L.; Lin, Y.T.; Kuo, Y.C.; Leu, Y.L. Chemical constituents from Lobelia chinensis and their anti-virus and ant-inflammatory bioactivities. Arch. Pharm. Res. 2011, 34, 715–722. [Google Scholar] [CrossRef]
- Catalan, C.A.N.; de Heluani, C.S.; Kotowicz, C.; Gedris, T.E.; Herz, W. A linear sesterterpene, two squalene derivative and two peptide derivatives from Croton hieronymi. Phytochemistry 2003, 64, 625–629. [Google Scholar] [CrossRef]
- Wu, T.S.; Chan, Y.Y.; Liou, M.J.; Lin, F.W.; Shi, L.S.; Chen, K.T. Platelet aggregation inhibitor from Murraya euchrestifolia. Phytother. Res. 1998, 12, S80–S82. [Google Scholar] [CrossRef]
- Lundkvist, G.B.; Sellix, M.T.; Nygard, M.; Davis, E.; Straume, M.; Kristensson, K.; Block, G.D. Clock gene expression during chronic inflammation induced by infection with Trypanosoma brucei brucei in rats. J. Biol. Rhythms. 2010, 2, 92–102. [Google Scholar]
- Nwagwu, M.; Inyang, A.L.; Molokwu, R.I.; Essen, E.M. Platelet aggregating activity of released factor(s) from Trypanosoma brucei brucei. Afr. J. Med. Sci. 1989, 4, 283–287. [Google Scholar]
- Balunas, M.J.; Su, B.; Riswan, S.; Fong, H.H.S.; Brueggemeier, R.W.; Pezzuto, J.M.; Kinghorn, A.D. Isolation and characterisation of aromatase inhibitor from Brassaiopsis glomerulata (Araliaceae). Phytochem. Lett. 2009, 2, 29–33. [Google Scholar] [CrossRef]
- Baltz, T.; Baltz, D.; Giroud, C.; Crockett, J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar]
- Orhan, I.; Sener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs 2010, 8, 47–58. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nwodo, N.J.; Okoye, F.B.C.; Lai, D.; Debbab, A.; Brun, R.; Proksch, P. Two Trypanocidal Dipeptides from the Roots of Zapoteca portoricensis (Fabaceae). Molecules 2014, 19, 5470-5477. https://doi.org/10.3390/molecules19055470
Nwodo NJ, Okoye FBC, Lai D, Debbab A, Brun R, Proksch P. Two Trypanocidal Dipeptides from the Roots of Zapoteca portoricensis (Fabaceae). Molecules. 2014; 19(5):5470-5477. https://doi.org/10.3390/molecules19055470
Chicago/Turabian StyleNwodo, Ngozi Justina, Festus Basden C. Okoye, Daowan Lai, Abdessamad Debbab, Reto Brun, and Peter Proksch. 2014. "Two Trypanocidal Dipeptides from the Roots of Zapoteca portoricensis (Fabaceae)" Molecules 19, no. 5: 5470-5477. https://doi.org/10.3390/molecules19055470
APA StyleNwodo, N. J., Okoye, F. B. C., Lai, D., Debbab, A., Brun, R., & Proksch, P. (2014). Two Trypanocidal Dipeptides from the Roots of Zapoteca portoricensis (Fabaceae). Molecules, 19(5), 5470-5477. https://doi.org/10.3390/molecules19055470