In-Silico Analyses of Sesquiterpene-Related Compounds on Selected Leishmania Enzyme-Based Targets
Abstract
:1. Introduction
2. Results and Discussion
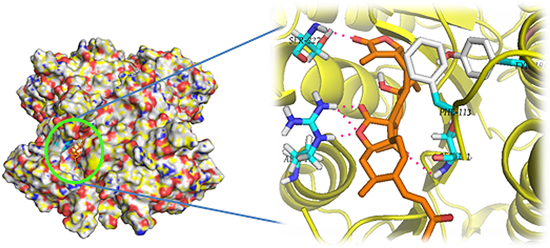
2.1. Molecular Docking of Sesquiterpenes
| Enzyme | PDB ID a | Resolution [Å] | Source | Abbreviation | Reference b |
|---|---|---|---|---|---|
| Pteridine reductase | 2QHX | 2.61 | L. major | LmPTR1 | [17] |
| Cysteine synthase | 4AIR | 1.80 | L. major | LdNMT | [10] |
| N-Myristoyl transferase | 2WUU | 1.42 | L. donovani | LmCS | [13] |
| Trypanothione synthetase | 2VOB | 2.30 | L. major | LmTryS | [11] |




| No | LmPTR1 | LmCS | LdNMT | LmTryS | No | LmPTR1 | LmCS | LdNMT | LmTryS | No | LmPTR1 | LmCS | LdNMT | LmTryS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sesquiterpene coumarins | drimanes | germacranolides cont. | ||||||||||||
| 1 | −9.9 | −11.2 | −6.6 | −8.9 | 45 | −6.9 | −7.6 | −4.5 | −7.0 | 86 | −8.0 | −9.0 | −6.2 | −8.3 |
| 2 | −10.0 | −11.8 | −6.8 | −8.5 | 46 | −8.9 | −9.9 | −5.9 | −7.9 | 87 | −8.1 | −9.5 | −5.5 | −7.7 |
| 3 | −7.8 | −10.0 | −5.6 | −7.4 | 47 | −8.6 | −9.9 | −6.1 | −8.3 | 88 | −8.1 | −8.7 | −5.2 | −7.5 |
| 4 | −7.4 | −9.5 | −5.1 | −7.9 | 48 | −8.9 | −10.8 | −5.5 | −7.8 | 89 | −8.4 | −9.0 | −5.7 | −8.7 |
| 5 | −7.5 | −10.0 | −4.9 | −8.2 | pseudoguaianolides | 90 | −8.0 | −9.6 | −5.7 | −7.9 | ||||
| 6 | −9.5 | −10.2 | −6.4 | −8.4 | 49 | −8.6 | −8.5 | −5.1 | −7.9 | 91 | −8.2 | −9.0 | −6.3 | −7.4 |
| 7 | −8.9 | −10.2 | −6.3 | −8.4 | 50 | −7.9 | −8.5 | −5.2 | −7.0 | 92 | −7.6 | −8.7 | −5.8 | −7.4 |
| 8 | −9.2 | −10.2 | −6.0 | −8.1 | 51 | −8.1 | −9.6 | −5.6 | −8.6 | 93 | −7.7 | −8.9 | −5.8 | −7.3 |
| 9 | −8.6 | −10.7 | −6.2 | −8.2 | 52 | −8.2 | −10.5 | −5.7 | −7.9 | 94 | −8.1 | −8.6 | −5.7 | −7.7 |
| agarofurans | 53 | −8.0 | −8.5 | −5.5 | −7.3 | 95 | −7.7 | −8.7 | −5.9 | −7.5 | ||||
| 10 | −8.8 | −8.5 | −5.5 | −7.0 | 54 | −8.3 | −9.2 | −6.1 | −7.6 | 96 | −8.6 | −8.7 | −5.7 | −7.1 |
| 11 | −7.5 | −7.4 | −4.7 | −6.6 | 55 | −8.3 | −8.9 | −5.4 | −7.6 | 97 | −8.8 | −9.2 | −5.5 | −7.3 |
| 12 | −7.8 | −7.6 | −5.1 | −6.5 | 56 | −8.2 | −9.7 | −5.7 | −8.0 | 98 | −8.3 | −9.0 | −5.1 | −7.2 |
| 13 | −7.4 | −8.9 | −5.1 | −3.2 | 57 | −7.8 | −9.1 | −5.7 | −7.3 | eudesmanolides | ||||
| 14 | −7.2 | −9.0 | −5.2 | −3.1 | 58 | −8.1 | −8.5 | −5.9 | −7.5 | 99 | −7.8 | −8.4 | −5.1 | −7.1 |
| 15 | −8.2 | −8.1 | −5.0 | −5.6 | 59 | −8.3 | −9.4 | −5.9 | −8.0 | 100 | −7.8 | −8.4 | −5.1 | −7.1 |
| 16 | −8.3 | −9.8 | −4.9 | −5.3 | 60 | −8.0 | −8.7 | −6.4 | −8.1 | 101 | −8.1 | −8.1 | −5.5 | −7.8 |
| 17 | −7.9 | −8.7 | −5.6 | −5.6 | 61 | −8.2 | −9.1 | −6.2 | −8.0 | 102 | −7.9 | −8.1 | −5.9 | −6.9 |
| 18 | −8.3 | −7.7 | −5.3 | −6.0 | 62 | −7.9 | −8.5 | −5.3 | −7.4 | 103 | −7.9 | −8.4 | −5.2 | −6.7 |
| 19 | −9.2 | −8.3 | −5.8 | −6.8 | 63 | −8.3 | −10.5 | −6.2 | −8.3 | 104 | −8.4 | −9.0 | −5.2 | −7.1 |
| 20 | −9.2 | −10.4 | −6.7 | −6.1 | 64 | −8.9 | −9.2 | −5.7 | −8.6 | 105 | −8.0 | −9.4 | −5.7 | −7.5 |
| 21 | −9.2 | −10.7 | −6.7 | −5.9 | 65 | −8.0 | −8.8 | −5.8 | −7.9 | 106 | −7.8 | −8.8 | −5.6 | −7.1 |
| 22 | −9.0 | −10.2 | −6.9 | −6.5 | 66 | −8.8 | −8.7 | −6.0 | −7.7 | miscellaneous | ||||
| 23 | −8.7 | −10.4 | −5.8 | −5.6 | 67 | −8.3 | −9.0 | −5.4 | −7.4 | 107 | −8.1 | −9.9 | −5.2 | −7.6 |
| 24 | −9.0 | −11.9 | −5.6 | −7.4 | guaianolides | 108 | −7.8 | −9.6 | −5.8 | −8.0 | ||||
| 25 | −8.7 | −10.8 | −6.7 | −5.3 | 68 | −8.1 | −9.8 | −5.5 | −7.4 | 109 | −7.3 | −9.5 | −5.0 | −7.9 |
| 26 | −8.9 | −9.4 | −5.9 | −8.5 | 69 | −7.8 | −8.9 | −5.8 | −7.8 | 110 | −6.5 | −8.5 | −3.8 | −6.9 |
| 27 | −8.6 | −10.1 | −6.9 | −6.0 | 70 | −8.0 | −9.7 | −5.8 | −7.0 | 111 | −8.8 | −9.4 | −5.3 | −7.8 |
| 28 | −9.4 | −10.8 | −6.5 | −8.5 | xanthanolides | 112 | −7.4 | −7.9 | −5.2 | −7.0 | ||||
| 29 | −8.6 | −11.4 | −5.5 | −7.7 | 71 | −7.2 | −9.4 | −5.4 | −6.8 | 113 | −6.9 | −8.8 | −5.2 | −6.7 |
| 30 | −8.9 | −12.1 | −5.8 | −7.0 | 72 | −7.6 | −8.5 | −5.8 | −7.2 | 114 | −7.1 | −9.1 | −5.0 | −7.1 |
| 31 | −8.8 | −11.4 | −5.9 | −8.5 | 73 | −7.8 | −8.9 | −5.2 | −7.4 | 115 | −7.0 | −9.6 | −5.0 | −6.7 |
| 32 | −9.3 | −10.2 | −5.8 | −8.7 | 74 | −7.5 | −7.9 | −5.1 | −7.1 | 116 | −7.7 | −8.1 | −4.7 | −6.9 |
| 33 | −8.6 | −9.8 | −6.0 | −7.6 | 75 | −10.6 | −11.4 | −6.5 | −0.5 | 117 | −6.5 | −8.5 | −4.7 | −6.7 |
| 34 | −8.9 | −11.2 | −6.1 | −8.7 | 76 | −10.6 | −9.4 | −6.4 | −3.3 | 118 | −8.7 | −9.2 | −5.7 | −7.6 |
| 35 | −9.3 | −10.6 | −6.7 | −7.0 | 77 | −8.0 | −9.5 | −4.9 | −7.3 | 119 | −7.8 | −8.4 | −5.6 | −7.6 |
| 36 | −9.2 | −9.5 | −6.1 | −7.2 | 78 | −7.5 | −8.6 | −5.5 | −7.4 | 120 | −8.0 | −9.0 | −5.4 | −7.2 |
| 37 | −8.8 | −9.5 | −6.0 | −8.4 | germacranolides | 121 | −8.1 | −7.5 | −4.9 | −7.1 | ||||
| 38 | −8.9 | −10.0 | −6.7 | −6.6 | 79 | −7.7 | −8.6 | −5.8 | −7.4 | 122 | −8.3 | −9.2 | −5.8 | −7.7 |
| 39 | −9.5 | −9.4 | −6.9 | −6.1 | 80 | −8.1 | −9.3 | −5.6 | −8.5 | 123 | −7.9 | −8.6 | −5.2 | −7.7 |
| 40 | −9.3 | −10.4 | −6.8 | −5.9 | 81 | −8.5 | −9.1 | −6.4 | −6.7 | known inhibitors b | ||||
| 41 | −7.7 | −7.0 | −4.7 | −6.6 | 82 | −8.3 | −9.7 | −5.5 | −7.8 | I1 | −10.2 | − | − | − |
| 42 | −6.2 | −6.3 | −5.5 | −7.0 | 83 | −8.0 | −10.6 | −5.7 | −8.3 | I2 | − | −9.6 | − | − |
| 43 | −7.3 | −7.1 | −6.1 | −5.3 | 84 | −8.4 | −8.9 | −5.3 | −8.2 | I3 | − | − | −8.0 | − |
| 44 | −7.8 | −6.8 | −5.1 | −5.6 | 85 | −8.3 | −8.8 | −5.8 | −8.1 | I4 | − | − | − | −8.2 |
| Sesquiterpene−type skeletal | LmPTR1 | LmCS | LdNMT | LmTryS | ||||
|---|---|---|---|---|---|---|---|---|
| MDE a | %RSD b | MDE a | %RSD b | MDE a | %RSD b | MDE a | %RSD b | |
| sesquiterpene coumarins | −8.8 | 11.4 | −10.4 | 6.7 | −6.0 | 11.0 | −8.2 | 5.1 |
| agarofurans | −8.5 | 9.0 | −9.5 | 16.1 | −5.9 | 11.5 | −6.6 | 20.6 |
| drimanes | −8.3 | 11.5 | −9.6 | 14.3 | −5.5 | 12.9 | −7.8 | 7.0 |
| pseudoguaianolides | −8.2 | 3.5 | −9.1 | 6.7 | −5.7 | 6.2 | −7.8 | 5.9 |
| guaianolides | −8.0 | 1.9 | −9.5 | 5.2 | −5.7 | 3.0 | −7.4 | 5.4 |
| xanthanolides | −8.4 | 16.9 | −9.2 | 11.4 | −5.6 | 10.5 | −5.9 | 43.8 |
| germacranolides | −8.1 | 3.9 | −9.1 | 5.3 | −5.7 | 5.8 | −7.7 | 6.7 |
| eudesmanolides | −8.0 | 2.6 | −8.6 | 5.3 | −5.4 | 5.6 | −7.2 | 4.8 |
| miscellaneous | −7.6 | 9.0 | −8.9 | 7.5 | −5.1 | 9.5 | −7.3 | 6.2 |







| Compound | LogP a | MR b | MW c | MF d | PSA e | H-D f | H-A g | RB h |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.50 | 110.2 | 382.5 | C24H30O4 | 52.6 | 0 | 4 | 3 |
| 2 | 4.78 | 112.2 | 382.5 | C24H30O4 | 55.8 | 1 | 4 | 3 |
| 22 | 2.03 | 149.7 | 595.6 | C32H37NO10 | 147.6 | 1 | 11 | 10 |
| 24 | 3.48 | 152.7 | 578.6 | C33H38O9 | 128.6 | 2 | 9 | 9 |
| 27 | 2.03 | 149.7 | 595.6 | C32H37NO10 | 147.6 | 1 | 11 | 10 |
| 29 | 4.61 | 151.5 | 562.7 | C33H38O8 | 108.4 | 1 | 8 | 9 |
| 30 | 4.61 | 151.5 | 562.7 | C33H38O8 | 108.4 | 1 | 8 | 9 |
| 31 | 3.22 | 120.5 | 442.5 | C26H34O6 | 82.1 | 1 | 6 | 6 |
| 39 | 5.06 | 168.7 | 641.7 | C37H39NO9 | 127.3 | 0 | 10 | 11 |
| 51 | 1.08 | 70.6 | 262.3 | C15H18O4 | 63.6 | 1 | 4 | 0 |
| 63 | 1.70 | 95.6 | 364.4 | C20H28O6 | 93.1 | 2 | 6 | 3 |
| 64 | 1.70 | 95.6 | 364.4 | C20H28O6 | 93.1 | 2 | 6 | 3 |
| 75 | 1.74 | 139.4 | 508.6 | C30H36O7 | 107.0 | 1 | 7 | 3 |
| 76 | 0.82 | 137.9 | 524.6 | C30H36O8 | 119.5 | 1 | 8 | 3 |
| 81 | 1.22 | 99.0 | 380.4 | C20H28O7 | 110.1 | 2 | 7 | 4 |
| 83 | 1.86 | 97.6 | 364.4 | C20H28O6 | 89.9 | 1 | 6 | 4 |
3. Experimental Section
3.1. Protein Preparation
3.2. Preparation of the Ligands
3.3. Docking Analyses
3.4. Ligand Interactions
3.5. Drug-Like Properties Calculations
3.6. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loría-Cervera, E.N.; Andrade-Narváez, F.J. Animal models for the study of leishmaniasis immunology. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 1–11. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Arboleda, M.; Jaramillo, L.; Ortiz, D.; Díaz, A. Leishmaniasis cutánea y herpes zoster multidermatómico. Rev. Chil. Infectol. 2013, 30, 680–682. [Google Scholar] [CrossRef]
- Sundar, S.; Rai, M. Advances in the treatment of leishmaniasis. Curr. Opin. Infect. Dis. 2002, 15, 593–598. [Google Scholar] [CrossRef]
- Jebran, A.F.; Schleicher, U.; Steiner, R.; Wentker, P.; Mahfuz, F.; Stahl, H.C.; Amin, F.M.; Bogdan, C.; Stahl, K.W. Rapid healing of cutaneous Leishmaniasis by high-frequency electrocauterization and hydrogel wound care with or without DAC N-055: A randomized controlled phase II a trial in kabul. PLoS Negl. Trop. Dis. 2014, 8, e2694. [Google Scholar] [CrossRef]
- Barrett, M.P.; Mottram, J.C.; Goombs, G.H. Recent advances in identifying and validating drug targets in trypanosomes and leishmanias. Trends Microbiol. 1999, 7, 82–88. [Google Scholar] [CrossRef]
- Ong, H.B.; Sienkiewicz, N.; Wyllie, S.; Fairlamb, A.H. Dissecting the metabolic roles of pteridine reductase 1 in Trypanosoma. brucei and Leishmania. major. J. Biol. Chem. 2001, 286, 10429–10438. [Google Scholar]
- Kheirandish, F.; Bandehpour, M.; Haghighi, A.; Mahboudi, F.; Mohebali, M.; Kazemi, B. Inhibition of Leishmania major PTR1 Gene Expression by Antisense in Escherichia coli. Iran. J. Public Health 2012, 41, 65–71. [Google Scholar]
- Krauth-Siegel, R.L.; Comini, M.A. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim. Biophys. Acta 2008, 1780, 1236–1248. [Google Scholar]
- Fyfe, P.K.; Westrop, G.D.; Ramos, T.; Muller, S.; Coombs, G.H.; Hunter, W.N. Structure of Leishmania major cysteine synthase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 738–743. [Google Scholar] [CrossRef]
- Fyfe, P.K.; Oza, S.L.; Fairlamb, A.H.; Hunter, W.N. Leishmania trypanothione synthetase-amidase structure reveals a basis for regulation of conflicting synthetic and hydrolytic activities. J. Biol. Chem. 2008, 283, 17672–17680. [Google Scholar]
- Resh, M.D. Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1999, 1451, 1–16. [Google Scholar] [CrossRef]
- Brannigan, J.A.; Smith, B.A.; Yu, Z.; Brzozowski, A.M.; Hodgkinson, M.R.; Maroof, A.; Price, H.P.; Meier, F.; Leatherbarrow, R.J.; Tate, E.W.; et al. N-Myristoyltransferase from Leishmania donovani: Structural and functional characterisation of a potential drug target for visceral leishmaniasis. J. Mol. Biol. 2010, 396, 985–999. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected ideases-Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.A.; Biavatti, M.W.; Brun, R.; da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected ideases-Part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar]
- Ogungbe, I.V.; Setzer, W.N. In-silico Leishmania target selectivity of antiparasitic terpenoids. Molecules 2013, 18, 7761–7847. [Google Scholar] [CrossRef]
- Cavazzuti, A.; Paglietti, G.; Hunter, W.N.; Gamarro, F.; Piras, S.; Loriga, M.; Allecca, S.; Corona, P.; McLuskey, K.; Tulloch, L.; et al. Discovery of potent pteridine reductase inhibitors to guide antiparasite drug development. Proc. Natl. Acad. Sci. USA 2008, 105, 1448–1453. [Google Scholar] [CrossRef]
- Iranshahi, M.; Arfa, P.; Ramezani, M.; Jaafari, M.R.; Sadeghian, H.; Bassarello, C.; Piacente, S.; Pizza, C. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry 2007, 68, 554–561. [Google Scholar]
- Pérez-Victoria, J.M.; Tincusi, B.M.; Jiménez, I.A.; Bazzocchi, I.L.; Gupta, M.P.; Castanys, S.; Gamarro, F.; Ravelo, A.G. New natural sesquiterpenes as modulators of daunomycin resistance in a Multidrug-Resistant Leishmania tropica line. J. Med. Chem. 1999, 42, 4388–4393. [Google Scholar] [CrossRef]
- Torres-Romero, D.; Muñoz-Martínez, F.; Jiménez, I.A.; Castanys, S.; Gamarro, F.; Bazzocchi, I.L. Novel dihydro-β-agarofuran sesquiterpenes as potent modulators of human P-glycoprotein dependent multidrug resistance. Org. Biomol. Chem. 2009, 7, 5166–5172. [Google Scholar] [CrossRef]
- Torres-Romero, D.; Jiménez, I.A.; Rojas, R.; Gilman, R.H.; López, M.; Bazzocchi, I.L. Dihydro-β-agarofuran sesquiterpenes isolated from Celastrus vulcanicola as potential anti-Mycobacterium tuberculosis multidrug-resistant agents. Bioorg. Med. Chem. 2011, 19, 2182–2189. [Google Scholar] [CrossRef]
- Delgado-Méndez, P.; Herrera, N.; Chávez, H.; Estévez-Braun, A.; Ravelo, A.G.; Cortes, F.; Castanys, S.; Gamarro, F. New terpenoids from Maytenus. apurimacensis as MDR reversal agents in the parasite Leishmania. Bioorg. Med. Chem. 2008, 16, 1425–1430. [Google Scholar] [CrossRef]
- Santos, V.A.F.F.M.; Regasini, L.O.; Nogueira, C.R.; Passerini, G.D.; Martinez, I.; Bolzani, V.S.; Graminha, M.A.S.; Cicarelli, R.M.B.; Furlan, M. Antiprotozoal sesquiterpene pyridine alkaloids from Maytenus ilicifolia. J. Nat. Prod. 2012, 75, 991–995. [Google Scholar] [CrossRef]
- Claudino, V.D.; da Silva, K.C.; Cechinel Filho, V.; Yunes, R.A.; Delle Monache, F.; Giménez, A.; Salamanca, E.; Gutierrez-Yapu, D.; Malheiros, A. Drimanes from Drimys brasiliensis with leishmanicidal and antimalarial activity. Mem. Inst. Oswaldo Cruz 2013, 108, 140–144. [Google Scholar]
- Sülsen, V.P.; Frank, F.M.; Cazorla, S.I.; Anesini, C.A.; Malchiodi, E.L.; Freixa, B.; Vila, R.; Muschietti, L.V.; Martino, V.S. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae). Antimicrob. Agents Chemother. 2008, 52, 2415–2419. [Google Scholar] [CrossRef]
- Barrera, P.; Sülsen, V.P.; Lozano, E.; Rivera, M.; Beer, M.F.; Tonn, C.; Martino, V.S.; Sosa, M. A natural sesquiterpene lactones induce oxidative stress in Leishmania mexicana. Evid. Based. Complement. Alternat. Med. 2013, 2013, 163404. [Google Scholar]
- Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative structure—antiprotozoal activity relationships of sesquiterpene lactones. Molecules 2009, 14, 2062–2076. [Google Scholar] [CrossRef]
- Fournet, A.; Muñoz, V.; Roblot, F.; Hocquemiller, R.; Cavé, A.; Gantier, J.-C. Antiprotozoal activity of dehydrozaluzanin C, a sesquiterpene lactone isolated from Munnozia maronii (Asteraceae). Phytoterapy. Res. 1993, 7, 111–115. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Cortez, D.A.; Violin, T.Y.; Dias Filho, B.P.; Nakamura, C.V.; Ueda-Nakamura, T.; Ferreira, I.C.P. Antileishmanial activity of a guaianolide from Tanacetum parthenium (L.) Schultz Bip. Parasitol. Int. 2010, 59, 643–646. [Google Scholar] [CrossRef]
- Tiuman, T.S.; Ueda-Nakamura, T.; Cortez, D.A.G.; Filho, B.P.D.; Morgado-Díaz, J.A.; de Souza, W.; Nakamura, C.V. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob. Agents Chemother. 2005, 49, 176–182. [Google Scholar] [CrossRef]
- Berger, I.; Passreiter, C.M.; Cáceres, A.; Kubelka, W. Antiprotozoal activity of Neurolaena lobata. Phytother. Res. 2001, 15, 327–30. [Google Scholar] [CrossRef]
- Passreiter, C.M.; Wendisch, D.; Gondol, D. Sesquiterpene lactones from Neurolaena lobata. Phytochemistry 1995, 39, 133–137. [Google Scholar] [CrossRef]
- Sharma, U.; Singh, D.; Kumar, P.; Dobhal, M.P.; Singh, S. Antiparasitic activity of plumericin & isoplumericin isolated from Plumeria bicolor against Leishmania donovani. Indian J. Med. Res. 2011, 709–716. [Google Scholar]
- Savoia, D.; Avanzini, C.; Allice, T.; Callone, E.; Guella, G.; Dini, F. Antimicrobial activity of euplotin C, the sesquiterpene taxonomic marker from the marine ciliate Euplotes crassus. Antimicrob. Agents Chemother. 2004, 48, 3828–3833. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Morillas-Márquez, F.; Martín-Sánchez, J.; Valero-López, A.; Navarro-Moll, M.C. Activity of (−)-α-bisabolol against Leishmania infantum promastigotes. Phytomedicine 2010, 17, 279–281. [Google Scholar] [CrossRef]
- Rojas-Silva, P.; Graziose, R.; Vesely, B.; Poulev, A.; Mbeunkui, F.; Grace, M.H.; Kyle, D.E.; Lila, M.A.; Raskin, I. Leishmanicidal activity of a daucane sesquiterpene isolated from Eryngium foetidum. Pharm. Biol. 2014, 52, 398–401. [Google Scholar] [CrossRef]
- Dos Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Filho, B.P.D.; Sudatti, D.B.; Bianco, E.M.; Pereira, R.C.; Nakamura, C.V. Effect of elatol, isolated from red seaweed Laurencia dendroidea, on Leishmania amazonensi. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef]
- Gul, W.; Hammond, N.L.; Yousaf, M.; Peng, J.; Holley, A.; Hamann, M.T. Chemical transformation and biological studies of marine sesquiterpene (S)-(+)-curcuphenol and its analogs. Biochim. Biophys. Acta 2007, 1770, 1513–1519. [Google Scholar] [CrossRef]
- Odonne, G.; Herbette, G.; Eparvier, V.; Bourdy, G.; Rojas, R.; Sauvain, M.; Stien, D. Antileishmanial sesquiterpene lactones from Pseudelephantopus spicatus, a traditional remedy from the Chayahuita Amerindians (Peru), Part III. J. Ethnopharmacol. 2011, 137, 875–879. [Google Scholar] [CrossRef]
- Ganfon, H.; Bero, J.; Tchinda, A.T.; Gbaguidi, F.; Gbenou, J.; Moudachirou, M.; Frédérich, M.; Quetin-Leclercq, J. Antiparasitic activities of two sesquiterpenic lactones isolated from Acanthospermum hispidum D.C. J. Ethnopharmacol. 2012, 141, 411–417. [Google Scholar] [CrossRef]
- Karioti, A.; Skaltsa, H.; Kaiser, M.; Tasdemir, D. Trypanocidal, leishmanicidal and cytotoxic effects of anthecotulide-type linear sesquiterpene lactones from Anthemis auriculata. Phytomedicine 2009, 16, 783–787. [Google Scholar] [CrossRef]
- Chollet, C.; Crousse, B.; Bories, C.; Bonnet-Delpon, D.; Loiseau, P.M. In vitro antileishmanial activity of fluoro-artemisinin derivatives against Leishmania donovani. Biomed. Pharmacother. 2008, 62, 462–465. [Google Scholar] [CrossRef]
- Picman, A.K.; Rodríguez, E.; Towers, G.H.N. Formation of adducts of parthenin and related sesquiterpene lactones with cysteine and glutathione. Chem. Biol. Interactions 1979, 28, 83–89. [Google Scholar] [CrossRef]
- Salapovic, H.; Geier, J.; Reznicek, G. Quantification of sesquiterpene lactones in asteraceae plant extracts: Evaluation of their allergenic potential. Sci. Pharm. 2013, 81, 807–818. [Google Scholar] [CrossRef]
- Fuchino, H.; Koide, T.; Takahashi, M.; Sekita, S.; Satake, M. New sesquiterpene lactones from Elephantopus mollis and their leishmanicidal activities. Planta Med. 2001, 67, 647–53. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev.Drug Discov. 2004, 3, 935–949. [Google Scholar]
- Vilar, S.; Costanzi, S. Predicting biological activities through QSAR analysis and Docking-based scoring. Methods Mol. Biol. 2012, 914, 271–284. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Delivery Rev. 1997, 23, 4–25. [Google Scholar]
- Discovery Studio Modeling Environment; Release 2.5; Accelrys Software Inc: San Diego, CA, USA, 2013.
- Spartan ‘08 V1.1.1; Wavefunction, Inc.: Irvine, CA, USA, 2008.
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2012. ISBN 3–900051–07–0. Available online: http://www.R-project.org/ (accessed on 16 March 2014).
- Research Network Natural Products against Neglected Diseases (ResNet NPND); Westfälische Wilhelms-Universität Münster, 2011. Available online: http://www.uni-muenster.de/Chemie.pb/forschen/ResNetNPND/ (accessed on 16 March 2014).
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bernal, F.A.; Coy-Barrera, E. In-Silico Analyses of Sesquiterpene-Related Compounds on Selected Leishmania Enzyme-Based Targets. Molecules 2014, 19, 5550-5569. https://doi.org/10.3390/molecules19055550
Bernal FA, Coy-Barrera E. In-Silico Analyses of Sesquiterpene-Related Compounds on Selected Leishmania Enzyme-Based Targets. Molecules. 2014; 19(5):5550-5569. https://doi.org/10.3390/molecules19055550
Chicago/Turabian StyleBernal, Freddy A., and Ericsson Coy-Barrera. 2014. "In-Silico Analyses of Sesquiterpene-Related Compounds on Selected Leishmania Enzyme-Based Targets" Molecules 19, no. 5: 5550-5569. https://doi.org/10.3390/molecules19055550
APA StyleBernal, F. A., & Coy-Barrera, E. (2014). In-Silico Analyses of Sesquiterpene-Related Compounds on Selected Leishmania Enzyme-Based Targets. Molecules, 19(5), 5550-5569. https://doi.org/10.3390/molecules19055550