Bioactive Secondary Metabolites from Phomopsis sp., an Endophytic Fungus from Senna spectabilis
Abstract
:1. Introduction

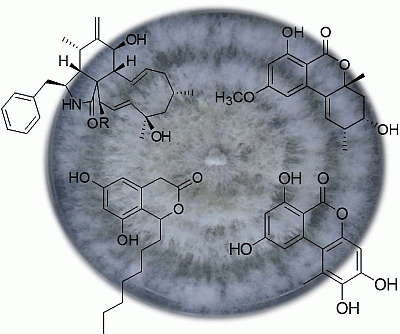
2. Results and Discussion
| 7 | ||
|---|---|---|
| Position | δH,mult. (J in Hz) | δC |
| 1 | - | 122.0 |
| 2 | - | 141.7 |
| 3 | - | 146.5 |
| 4 | 6.68 ( s) | 100.7 |
| 5 | - | * |
| 6 | - | * |
| 6a | - | 97.4 |
| 7 | - | 164.4 |
| 8 | 6.34 ( d, 2.5 Hz) | 100.5 |
| 9 | - | 165.0 |
| 10 | 7.26 ( d, 2.5 Hz) | 104.1 |
| 10a | - | * |
| 10b | - | 109.0 |
| 1-CH3 | 2.58 ( s) | 18.8 |


| Compounds | Concentration (µmol L−1) | Viable cells (%) * | |
|---|---|---|---|
| 30 min | 60 min | ||
| Control | 99.0 ± 0.60 | 99.0 ± 1.41 | |
| 1 | 100 | 80.0 ± 2.80 | 50.0 ± 2.80 |
| 1 | 10 | 97.0 ± 0.70 | 97.0 ± 0.70 |
| 2 | 100 | 85.0 ± 1.40 | 60.0 ± 2.80 |
| 2 | 10 | 96.0 ± 0.70 | 96.0 ± 1.41 |
| 4 | 100 | 85.0 ± 3.50 | 50.0 ± 2.80 |
| 4 | 10 | 95.0 ± 1.41 | 95.0 ± 0.70 |
| 5 | 100 | 70.0 ± 2.80 | 45.0 ± 2.80 |
| 5 | 10 | 96.0 ± 0.70 | 96.0 ± 0.70 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Isolation and Identification of the Endophytes
3.4. Fermentation, Extraction and Isolation
3.5. Biological Activity
3.5.1. The Reactive Oxygen Species (ROS) Inhibitory Activity was Measured Using a Cellular Assay
3.5.2. Myeloperoxidase (MPO) Inhibitory Activity
3.5.3. Antioxidant Capacity
3.5.4. Cytotoxic Activity
3.5.5. Antifungal Activity
3.5.6. Acetylcholinesterase Inhibitory Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aly, H.A.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar]
- Gunatilaka, A.A.L. Natural Products from plant-associated microorganisms: Distribution, structutal diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef]
- Jalgaonwala, R.E.; Mohite, B.V.; Mahajan, R.T. A review: Natural products from plant associated endophytic fungi. J. Microbiol. Biotech. Res. 2011, 1, 21–32. [Google Scholar]
- Magnani, R.F.; de Souza, G.D.; Rodrigues-Filho, E. Analysis of alternariol and alternariol monomethyl ether on flavedo and albedo tissues of tangerines (Citrus reticulata) with symptoms of Alternaria brown spot. J. Agric. Food Chem. 2007, 55, 4980–4986. [Google Scholar] [CrossRef]
- Watanabe, I.; Kakishima, M.; Adachi, Y.; Nakajima, H. Potencial mycotoxin productivity of Alternaria alternata isolated from garden trees. Mycotoxins 2007, 57, 3–9. [Google Scholar]
- Bolzani, V.S.; Trevisan, L.M.V.; Young, M.C.M. Caffeic acid esters and triterpenes of Alibertia macrophyla. Phytochemistry 1991, 30, 2089–2091. [Google Scholar]
- Young, M.C.M.; Braga, M.R.; Dietrich, S.M.C.; Gottlieb, H.E.; Trevisan, L.M.V.; Bolzani, V.S. Fungitoxic non-glycosidic iridoids from Alibertia macrophylla. Phytochemistry 1992, 31, 3433–3435. [Google Scholar] [CrossRef]
- Silva, V.C.; Faria, A.O.; Bolzani, V.S.; Lopes, M.N. A new ent-kaurane diterpene from stems of Alibertia macrophylla K. Schum. (Rubiaceae). Helv. Chim. Acta 2007, 90, 1781–1785. [Google Scholar] [CrossRef]
- Junior, C.V.; Pivatto, M.; Rezende, A.; Hamerski, L.; Silva, D.H.S.; Bolzani, V.S. (–)-7-Hydroxycassine: A new 2,6-dialkylpiperidin-3-ol alkaloid and other constituents isolated from flowers and fruits of Senna spectabilis (Fabaceae). J. Braz. Chem. Soc. 2013, 24, 230–235. [Google Scholar]
- Izawa, Y.; Hirose, T.; Shimizu, T.; Koyama, K.; Natori, S. Six new 10-phenyl-[11]cytochalasans, cytochalasins N-S from Phompsis sp. Tetrahedron 1989, 45, 2323–2335. [Google Scholar] [CrossRef]
- Tao, Y.; Zeng, X.; Mou, C.; Li, J.; Cai, X.; She, Z.; Zhou, S.; Lin, Y. 1H and 13C NMR assignments of three nitrogen containing compounds from the mangrove endophytic fungus (ZZF08). Magn. Reson. Chem. 2008, 46, 501–505. [Google Scholar] [CrossRef]
- Ondeyka, J.; Hensens, O.D.; Zink, D.; Ball, R.; Lingham, R.B.; Bills, G.; Dombrowski, A.; Goetz, M. L-696,474, a novel cytochalasin as an inhibitor of HIV-1-protease. II. Isolation and structure. J. Antibiot. 1992, 45, 679–685. [Google Scholar] [CrossRef]
- Bradburn, N.; Coker, R.D.; Blunden, G.; Turner, C.H.; Crabb, T.A. 5'-Epiltenuene and neoaltenuene, dibenzo-a-pyrones from Alternaria alternata cultured on rice. Phytochemistry 1994, 35, 665–669. [Google Scholar] [CrossRef]
- Jiao, P.; Gloer, J.B.; Campbell, J.; Shearer, C.A. Altenuene derivatives from an unidentified freshwater fungus in the family Tubeufiaceae. J. Nat. Prod. 2006, 69, 612–615. [Google Scholar] [CrossRef]
- Gu, W. Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Malus halliana. World J. Microbiol. Biotechnol. 2009, 25, 1677–1683. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-ebel, R.; Indriani, I.D.; Wray, V.; Muller, W.E.G.; Totzke, F.; Zirrgiebel, U.; Schachtele, C.; Kubbutat, M.H.G.; Lin, W.H.; et al. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection on its host plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar]
- Tan, N.; Tao, Y.; Pan, J.; Wang, S.; Xu, F.; She, Z.; Lin, Y.; Jones, E.B.G. Isolation, structure elucidation, and mutagenicity of four alternariol derivatives produced by the mangrove endophytic fungus N. 2240. Chem. Nat. Comp. 2008, 43, 296–300. [Google Scholar]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Guan, H.; Edrada, R.; Muller, V.E.G.; Bayer, M.; Lin, W.; Wu, J.; et al. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophoramucronata. Bioorg. Med. Chem. 2009, 17, 7362–7367. [Google Scholar]
- Brady, S.F.; Wagenaar, M.M.; Singh, M.P.; Janso, F.E.; Cardy, J. The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Organic Letters 2000, 25, 4043–4046. [Google Scholar]
- Pfeiffer, E.; Schebb, N.H.; Podlech, J.; Metzler, M. Novel oxidative in vitro metabolites of the mycotoxins alternariol and alternariol methyl ether. Mol. Nutr. Food Res. 2007, 51, 307–316. [Google Scholar] [CrossRef]
- Almeida, A.C.; Marques, O.C.; Arslanian, C.; Condino-Neto, A.; Ximenes, V.F. 4-Fluoro-2-methoxyphenol, na apocynin analog with enhaced inhibitory effect on leukocyte oxidant production and phagocytosis. Eur. J. Pharmacol. 2011, 660, 445–453. [Google Scholar] [CrossRef]
- Jan, A.T.; Kamli, M.R.; Murtaza, I.; Singh, J.B.; Ali, A.; Haq, Q.M.R. Dietary flavonoid quercetin and associated health benefits — an overview. Food Rev. Int. 2010, 26, 302–317. [Google Scholar] [CrossRef]
- Ford, D.A. Lipid oxidation by hypochlorous acid: Chlorinated lipids in atherosclerosis and myocardial ischemia. Clin. Lipidol. 2010, 5, 835–852. [Google Scholar] [CrossRef]
- Summers, F.A.; Morgan, P.E.; Davies, M.J.; Hawkins, C.L. Identification of plasma proteins that are susceptible to thiol oxidation by hypochlorous acid and N-chloramines. Chem. Res. Toxicol. 2008, 21, 1832–1840. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxiase: Friend and foe. J. Leukocyte Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Xu, S.; Ge, H.M.; Song, Y.C.; Shen, Y.; Ding, H.; Tan, R.X. Cytotoxic cytochalasin metabolites of endophytic Endothia gyrosa. Chem. Biodivers. 2009, 6, 739–745. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, G.; Zhu, T.; Liu, R.; Wei, H.; Gu, Q. Bioactive cytochalasins from Aspergillus flavipes, an endophytic fungus associated with the mangrove plant Acanthus ilicifolius. Helv. Chim. Acta 2009, 92, 1538–1544. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycot. J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Kettle, A.J. Detection of 3-chlorotyrosine in proteins exposed to neutrophil oxidants. Meth. Enzymol. 1999, 300, 111–120. [Google Scholar] [CrossRef]
- Beers, R.J.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar]
- Silva, G.H.; Teles, H.L.; Zanardi, L.M.; Young, M.C.M.; Eberlin, M.N.; Haddad, R.; Pfenning, L.H.; Costa-Neto, C.; Castro-Gamboa, I.; Bolzani, V.S.; et al. Cadinane sesquiterpenoids of Phomopsis cassiae, an endophytic fungus associated with Cassia spectabilis (Leguminosae). Phytochemistry 2006, 67, 1964–1969. [Google Scholar] [CrossRef]
- English, D.; Andersen, B.R. Single-step separation of red blood cells, granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J. Immunol. Methods 1974, 5, 249–252. [Google Scholar]
- Kitagawa, R.R.; Raddi, M.S.G.; Khalil, N.M.; Vilegas, W.; Fonseca, L.M. Effect of the isocoumarin paepalantine on the luminol and lucigenin amplified chemiluminescence of rat neutrophils. Biol. Pharm. Bull. 2003, 26, 905–908. [Google Scholar] [CrossRef]
- Malle, E.; Furtmuller, P.G.; Sattler, W.; Obinger, C. Myeloperoxidase: A target for new drug development? Br. J. Pharmacol. 2007, 152, 838–854. [Google Scholar] [CrossRef]
- Zeraik, M.L; Ximenes, V.F.; Regasini, L.O.; Dutra, L.A.; Silva, D.H.; Fonseca, L.M.; Coelho, D.; Machado, S.A.; Bolzani, V.S. 4'-Aminochalcones as novel inhibitors of the chlorinating activity of myeloperoxidase. Curr. Med. Chem. 2012, 19, 5405–5413. [Google Scholar] [CrossRef]
- Ximenes, V.F.; Kanegae, M.P.; Rissato, S.R.; Galhiane, M.S. The oxidation of apocynin catalyzed by myeloperoxidase: Proposal for NADPH oxidase inhibition. Arch. Biochem. Biophys. 2007, 457, 134–141. [Google Scholar] [CrossRef]
- Tan, A.S.; Berridge, M.V. Superoxide produced by active neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: A simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J. Immunol. Methods 2000, 238, 59–68. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm.-Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zeraik, M.L.; Yariwake, J.H.; Wauters, J.N.; Tits, M.; Angenot, L. Analysis of passion fruit rinds (Passiflora edulis): Isoorientin quantification by HPTLC and evaluation of antioxidant (radical scavenging) capacity. Quim. Nova 2012, 35, 541–545. [Google Scholar] [CrossRef]
- Rahalison, L.; Hamburger, M.; Hostettmann, K.; Monod, M.; Frenk, E. A bioautographic agar overlay method for the detection of antifungal compounds from higher plants. Phytochem. Anal. 1991, 2, 199–203. [Google Scholar] [CrossRef]
- Marston, A.; Kissling, J.; Hostettmann, K. A rapid TCL bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002, 13, 51–54. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chapla, V.M.; Zeraik, M.L.; Ximenes, V.F.; Zanardi, L.M.; Lopes, M.N.; Cavalheiro, A.J.; Silva, D.H.S.; Young, M.C.M.; Fonseca, L.M.d.; Bolzani, V.S.; et al. Bioactive Secondary Metabolites from Phomopsis sp., an Endophytic Fungus from Senna spectabilis. Molecules 2014, 19, 6597-6608. https://doi.org/10.3390/molecules19056597
Chapla VM, Zeraik ML, Ximenes VF, Zanardi LM, Lopes MN, Cavalheiro AJ, Silva DHS, Young MCM, Fonseca LMd, Bolzani VS, et al. Bioactive Secondary Metabolites from Phomopsis sp., an Endophytic Fungus from Senna spectabilis. Molecules. 2014; 19(5):6597-6608. https://doi.org/10.3390/molecules19056597
Chicago/Turabian StyleChapla, Vanessa Mara, Maria Luiza Zeraik, Valdecir F. Ximenes, Lisinéia Maria Zanardi, Márcia N. Lopes, Alberto José Cavalheiro, Dulce Helena S. Silva, Maria Cláudia M. Young, Luiz Marcos da Fonseca, Vanderlan S. Bolzani, and et al. 2014. "Bioactive Secondary Metabolites from Phomopsis sp., an Endophytic Fungus from Senna spectabilis" Molecules 19, no. 5: 6597-6608. https://doi.org/10.3390/molecules19056597
APA StyleChapla, V. M., Zeraik, M. L., Ximenes, V. F., Zanardi, L. M., Lopes, M. N., Cavalheiro, A. J., Silva, D. H. S., Young, M. C. M., Fonseca, L. M. d., Bolzani, V. S., & Araújo, A. R. (2014). Bioactive Secondary Metabolites from Phomopsis sp., an Endophytic Fungus from Senna spectabilis. Molecules, 19(5), 6597-6608. https://doi.org/10.3390/molecules19056597