2.1. KYS05090 Induced Cell Death in A549 Cells
KYS05090 (
Figure 1) was prepared as described previously [
8]. To investigate the cytotoxic effect of KYS05090, viable A549 cell numbers were analyzed with the presence of different concentrations (0, 4, 6, 8, or 10 μM) of KYS05090 for 24 h by an MTT assay.
Figure 1.
Discovery of KYS05090 via intensive structure-activity relationship (SAR) study on 3,4-dihydroquinazoline derivatives.
Figure 1.
Discovery of KYS05090 via intensive structure-activity relationship (SAR) study on 3,4-dihydroquinazoline derivatives.
As shown in
Figure 2A, KYS05090 (IC
50: 6 μM) reduced cell viability in a dose-dependent manner. To identify the molecular mechanism underlying cell death induced by KYS05090, the translocation of phosphatidylserine was assessed using Annexin V and propidium iodide (PI) double staining by flow cytometry. As shown in
Figure 2B, KYS05090 (6 μM for 24 h) increased both non-apoptotic and apoptotic cell death by up to 55% and 24%, respectively.
Figure 2.
KYS05090 induced cell death in A549 cells. (A) A549 cells were treated with KYS05090 (0, 4, 6, 8, or 10 μM) for 24 h. Cell viability was determined through an MTT assay. Presented data are means ± S.D. for three independent experiments. *** p < 0.001 vs. the control group. (B) Cells were stained with Annexin V-FITC and PI and analyzed by FACS.
Figure 2.
KYS05090 induced cell death in A549 cells. (A) A549 cells were treated with KYS05090 (0, 4, 6, 8, or 10 μM) for 24 h. Cell viability was determined through an MTT assay. Presented data are means ± S.D. for three independent experiments. *** p < 0.001 vs. the control group. (B) Cells were stained with Annexin V-FITC and PI and analyzed by FACS.
2.2. KYS05090 Induced Autophagy and Apoptosis in A549 Cells
Autophagy is thought to be a cytoprotective process in starving cells. However, excess autophagy can induce type II programmed cell death (autophagy-associated cell death) [
10]. Because KYS05090 induced non-apoptotic cell death, its ability to induce autophagy-associated cell death was examined. LC3 is localized in the cytoplasm under normal conditions but is cleaved and lipidated to be turned into LC3 II and recruited to the autophagosomes during autophagy [
10]. Therefore, amount and localization of LC3 II is used as marker of autophagy induction. KYS05090 induced LC3-II conversion in A549 cells in a time-dependent manner (
Figure 3A). In addition, the autophagic flux was verified by the decreased expression of p62, which can be degraded by autophagy (
Figure 3A). To verify the involvement of autophagy in KYS05090-induced cell death, bafilomycin A1 was used at concentrations that significantly blocked the induction of autophagy. As shown in
Figure 3C, bafilomycin A1 markedly suppressed KYS05090-induced cell death. These observations indicate that KYS05090-induced cell death involves the autophagy-dependent pathway in A549 cells.
Annexin V/PI double-staining results indicate that KYS05090 mildly induced apoptosis. To characterize KYS05090-triggered apoptosis, the activation of caspase 3 in A549 cells by KYS05090 treatment was examined by western blotting. Pro-caspase 3 was activated after KYS05090 treatment, and this induction was accompanied by an increase in the cleavage of its substrate poly (ADP-ribose) polymerase (PARP) (
Figure 3B). To determine whether the activation of caspases is required for the induction of apoptosis by KYS05090, the broad caspase inhibitor (zVAD-fmk) was pretreated in A549 cells. As shown in
Figure 3D, zVAD-fmk significantly inhibited KYS05090-induced cell death. These results indicate that KYS05090 induced caspase-dependent apoptotic cell death as well as autophagy-associated cell death.
Figure 3.
KYS05090 induced autophagy-associated and apoptotic cell death in A549 cells. (A,B) A549 cells were treated with KYS05090 (6 μM) for indicated times. The expression of proteins were analyzed by western blot analysis. β-Actin was used as an internal control. The immunoblots shown are representative of three independent experiments. Density ratios versus β-actin were measured by densitometry. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the control group (C) A549 cells were pretreated with bafilomycin A1 (0.5 or 1 μM) for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. (D) A549 cells were pretreated with zVAD-fmk (20 μM) for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group, and * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the KYS05090-treated group.
Figure 3.
KYS05090 induced autophagy-associated and apoptotic cell death in A549 cells. (A,B) A549 cells were treated with KYS05090 (6 μM) for indicated times. The expression of proteins were analyzed by western blot analysis. β-Actin was used as an internal control. The immunoblots shown are representative of three independent experiments. Density ratios versus β-actin were measured by densitometry. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the control group (C) A549 cells were pretreated with bafilomycin A1 (0.5 or 1 μM) for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. (D) A549 cells were pretreated with zVAD-fmk (20 μM) for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group, and * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the KYS05090-treated group.
![Molecules 19 09864 g003]()
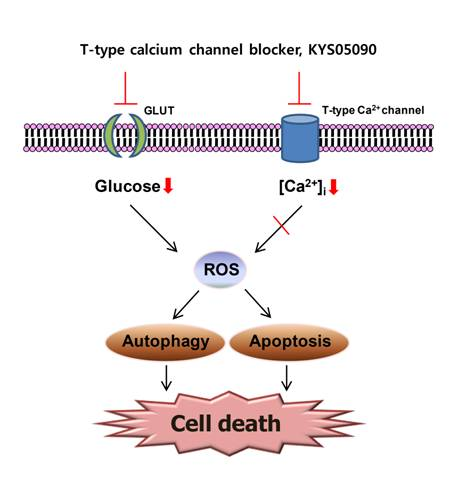
2.4. Reactive Oxygen Species (ROS) Were Involved in KYS05090-Induced Cell Death
Because ROS has been demonstrated to mediate autophagy as well as apoptosis [
12], its involvement in KYS05090-mediated cell death was examined. The level of ROS within cells was measured using a ROS-sensitive fluoromertric probe, DCFH-DA, and flow cytometry. As shown in
Figure 5A,B, cells treated with KYS05090 showed higher levels of ROS production in comparison to control cells, and the KYS05090-induced ROS generation was dose-dependently reduced by the antioxidant NAC or catalase. In addition, treatment with these antioxidants effectively blocked KYS05090-induced cell death, and noteworthy is that catalase was significantly more likely to prevent this cell death than NAC (
Figure 5C,D). Furthermore, catalase prevented KYS05090-induced LC3-II accumulation, p62 degradation, and PARP cleavage (
Figure 5E). These results suggest that ROS play a critical role in KYS05090-induced cell death.
Figure 4.
KYS05090 reduced [Ca2+]i but was not associated with KYS05090-induced cell death in A549 cells. (A) A549 cells were stained with Fluo-4 and treated with KYS05090 (1, 3, 6, 8, or 10 μM). [Ca2+]i were measured using a fluorescence reader. (B,C) A549 cells were pretreated with ionomycin (1, 5, or 10 μM) or KCl (30, 40, or 50 mM) for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group.
Figure 4.
KYS05090 reduced [Ca2+]i but was not associated with KYS05090-induced cell death in A549 cells. (A) A549 cells were stained with Fluo-4 and treated with KYS05090 (1, 3, 6, 8, or 10 μM). [Ca2+]i were measured using a fluorescence reader. (B,C) A549 cells were pretreated with ionomycin (1, 5, or 10 μM) or KCl (30, 40, or 50 mM) for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group.
2.5. KYS05090 Induced Autophagy and Apoptosis by Suppressing Glucose Uptake in A549 Cells
Several studies have reported that Ca
2+ channel antagonists inhibit glucose uptake and that glucose deprivation subsequently induces oxidative stress associated with aggresome formation and autophagy activation [
13,
14,
15]. In addition, ROS inhibition by catalase can prevent cell death induced by glucose deprivation [
16]. Therefore, the ability of KYS05090 to inhibit glucose uptake, as in other Ca
2+ channel antagonists, was examined, and the results indicate that KYS05090 dose-dependently inhibited glucose uptake in A549 cells (
Figure 6A). To determine whether KYS05090 would induce cell death through the inhibition of glucose uptake, A549 cells were pretreated with methyl pyruvate (cell permeable pyruvate). As shown in
Figure 6B, methyl pyruvate significantly inhibited KYS05090-induced cell death. In addition, methyl pyruvate prevented KYS05090-induced LC3-II accumulation, p62 degradation, and PARP cleavage (
Figure 6C). By contrast, sodium pyruvate (cell impermeable pyruvate) or glucose did not attenuate KYS05090-induced cell death (
Figure 6D,E). These results indicate that KYS05090-induced cell death was caused by the downregulation of glucose uptake.
Figure 5.
KYS05090 generated ROS, and antioxidants inhibited KYS05090-induced cell death. (A,B) A549 cells were pretreated with NAC or catalase for 30 min, followed by treatment with KYS05090 (6 μM) for 30 min and DCFH-DA (5 µM) for 30 min. Subsequently, the cells were collected and analyzed by FACS. (C,D) A549 cells were pretreated with NAC or catalase for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group, and * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the KYS05090-treated group. (E) A549 cells were pretreated with catalase for 30 min and then treated with KYS05090 (6 μM) for 24 h. Protein levels were analyzed by western blot analysis. β-Actin was used as an internal control. The immunoblots shown are representative of three independent experiments. Density ratios versus β-actin were measured by densitometry. # p < 0.05 vs. the control group, and * p < 0.05, ** p < 0.01 vs. the KYS05090-treated group.
Figure 5.
KYS05090 generated ROS, and antioxidants inhibited KYS05090-induced cell death. (A,B) A549 cells were pretreated with NAC or catalase for 30 min, followed by treatment with KYS05090 (6 μM) for 30 min and DCFH-DA (5 µM) for 30 min. Subsequently, the cells were collected and analyzed by FACS. (C,D) A549 cells were pretreated with NAC or catalase for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group, and * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the KYS05090-treated group. (E) A549 cells were pretreated with catalase for 30 min and then treated with KYS05090 (6 μM) for 24 h. Protein levels were analyzed by western blot analysis. β-Actin was used as an internal control. The immunoblots shown are representative of three independent experiments. Density ratios versus β-actin were measured by densitometry. # p < 0.05 vs. the control group, and * p < 0.05, ** p < 0.01 vs. the KYS05090-treated group.
![Molecules 19 09864 g005]()
It has been reported that glucose deprivation can generate ROS [
15]. To investigate the relationship between KYS05090-induced ROS generation and the inhibition of glucose uptake, KYS05090-induced ROS generation was determined after the treatment of A549 cells with methyl pyruvate. As shown in
Figure 6F, methyl pyruvate (1 mM) significantly reduced KYS05090-induced ROS generation. This indicates that KYS05090 generated ROS through the inhibition of glucose uptake. Methyl pyruvate (1 mM) prevented KYS05090-induced cell death more powerfully than 0.5 mM of methyl pyruvate (
Figure 6B). However, the difference in levels of p62 or ROS are less than cell death level (
Figure 6C,F). These results give a possibility that KYS05090-induced cell death may be partially related with autophagy and ROS generation.
Figure 6.
KYS05090 suppressed glucose uptake, and methyl pyruvate inhibited KYS05090-induced autophagy-associated and apoptotic cell death in A549 cells. (A) A549 cells (5 × 104/mL) were seeded in a 96-well plate and treated with KYS05090 (2, 4, or 6 μM) in the glucose- and serum-free culture medium. After 2 h, 100 µg/mL of 2-NBDG was added, and the plates were incubated for an additional 2 h. Fluorescence intensity was measured using a fluorescence plate reader. Apigenin was used as a positive control. Presented data are means ± S.D. for three independent experiments. * p < 0.05 and ** p < 0.01 vs. the control group. (B–E) A549 cells were pretreated with methyl pyruvate (0.5, 1, or 2 mM), sodium pyruvate (1, 5, or 10 mM), or glucose (0.5, 1, or 2 mM) for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. Protein levels were analyzed by western blot analysis. β-Actin was used as an internal control. The immunoblots shown are representative of three independent experiments. Density ratios versus β-actin were measured by densitometry. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group, and * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the KYS05090-treated group. (F) A549 cells were pretreated with methyl pyruvate (0.5, 1, or 2 mM) for 30 min, followed by treatment with KYS05090 (6 μM) for 30 min and DCFH-DA (5 µM) for 30 min. Then the cells were collected and analyzed by FACS. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group, and * p < 0.05 vs. the KYS05090-treated group.
Figure 6.
KYS05090 suppressed glucose uptake, and methyl pyruvate inhibited KYS05090-induced autophagy-associated and apoptotic cell death in A549 cells. (A) A549 cells (5 × 104/mL) were seeded in a 96-well plate and treated with KYS05090 (2, 4, or 6 μM) in the glucose- and serum-free culture medium. After 2 h, 100 µg/mL of 2-NBDG was added, and the plates were incubated for an additional 2 h. Fluorescence intensity was measured using a fluorescence plate reader. Apigenin was used as a positive control. Presented data are means ± S.D. for three independent experiments. * p < 0.05 and ** p < 0.01 vs. the control group. (B–E) A549 cells were pretreated with methyl pyruvate (0.5, 1, or 2 mM), sodium pyruvate (1, 5, or 10 mM), or glucose (0.5, 1, or 2 mM) for 30 min and then treated with KYS05090 (6 μM) for 24 h. The cells were stained with PI, and cell death was analyzed by FACS. Protein levels were analyzed by western blot analysis. β-Actin was used as an internal control. The immunoblots shown are representative of three independent experiments. Density ratios versus β-actin were measured by densitometry. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group, and * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the KYS05090-treated group. (F) A549 cells were pretreated with methyl pyruvate (0.5, 1, or 2 mM) for 30 min, followed by treatment with KYS05090 (6 μM) for 30 min and DCFH-DA (5 µM) for 30 min. Then the cells were collected and analyzed by FACS. Presented data are means ± S.D. for three independent experiments. # p < 0.05 vs. the control group, and * p < 0.05 vs. the KYS05090-treated group.
![Molecules 19 09864 g006]()