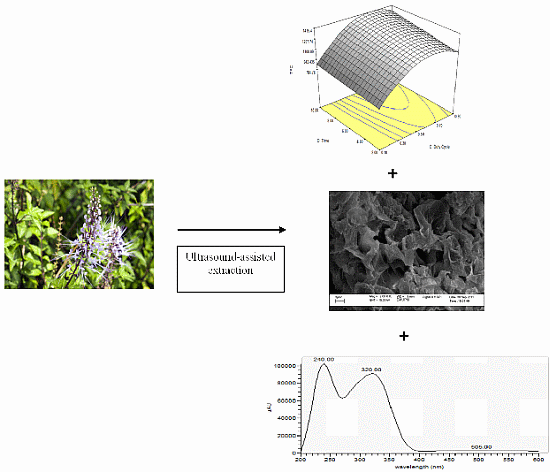
Ultrasound-Assisted Extraction of Antioxidants in Misai Kucing (Orthosiphon stamineus)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Modeling of UAE on Orthosiphon stamineus
2.2. Effect of Process Variables on Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)

| Standard Order a | Independent variables | Dependent variables (Responses) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 b | X2 c | X3 d | X4 e | Y1 f | Y2 g | Y3 h | Y4 i | |||||||||||||||||
| Expt. | Pred. | Expt. | Pred. | Expt. | Pred. | Expt. | Pred. | |||||||||||||||||
| 1 | 0.0 | 20.0 | 0.1 | 10.0 | 974.0 | 973.4 | 13,603.4 | 14,395.9 | 1952.3 | 1959.6 | 2219.8 | 2275.4 | ||||||||||||
| 2 | 100.0 | 20.0 | 0.1 | 2.0 | 265.3 | 270.3 | 4696.3 | 1180.7 | 1427.8 | 1497.6 | 1045.1 | 1037.8 | ||||||||||||
| 3 | 0.0 | 100.0 | 0.1 | 2.0 | 1048.9 | 1045.1 | 12,134.5 | 13,983.7 | 1952.3 | 1959.6 | 2216.1 | 2220.8 | ||||||||||||
| 4 | 100.0 | 100.0 | 0.1 | 10.0 | 248.3 | 237.4 | 8811.1 | 10,748.8 | 1571.6 | 1497.6 | 1880.2 | 1845.5 | ||||||||||||
| 5 | 0.0 | 20.0 | 0.9 | 2.0 | 949.9 | 937.9 | 10,730.0 | 15,920.2 | 1886.5 | 1888.2 | 2438.8 | 2469.7 | ||||||||||||
| 6 | 100.0 | 20.0 | 0.9 | 10.0 | 1056.5 | 1072.4 | 11,541.1 | 12,685.3 | 1957.0 | 1964.0 | 2284.9 | 2321.9 | ||||||||||||
| 7 | 0.0 | 100.0 | 0.9 | 10.0 | 1284.7 | 1283.5 | 38,108.8 | 25,488.3 | 1886.5 | 1888.2 | 1641.6 | 1645.1 | ||||||||||||
| 8 | 100.0 | 100.0 | 0.9 | 2.0 | 726.1 | 765.5 | 10,116.7 | 12,273.1 | 1959.4 | 1964.0 | 2277.5 | 2263.6 | ||||||||||||
| 9 | 50.0 | 60.0 | 0.5 | 6.0 | 1283.0 | 1260.2 | 36,170.9 | 37,703.9 | 1840.0 | 1827.3 | 1745.6 | 1727.5 | ||||||||||||
| 10 | 50.0 | 60.0 | 0.5 | 6.0 | 1269.2 | 1260.2 | 36,170.9 | 37,703.9 | 1840.0 | 1827.3 | 1785.3 | 1727.5 | ||||||||||||
| 11 | 0.0 | 20.0 | 0.1 | 2.0 | 1008.0 | 992.7 | 12,728.0 | 10,402.1 | 1950.8 | 1950.8 | 2219.2 | 2199.3 | ||||||||||||
| 12 | 100.0 | 20.0 | 0.1 | 10.0 | 469.0 | 454.7 | 6377.8 | 7167.2 | 1456.7 | 1488.8 | 1393.5 | 1433.6 | ||||||||||||
| 13 | 0.0 | 100.0 | 0.1 | 10.0 | 1216.8 | 1229.5 | 14,488.6 | 19,970.2 | 1950.8 | 1950.8 | 2175.6 | 2158.9 | ||||||||||||
| 14 | 100.0 | 100.0 | 0.1 | 2.0 | 247.1 | 256.7 | 5903.1 | 6755.1 | 1455.2 | 1488.8 | 1248.2 | 1311.7 | ||||||||||||
| 15 | 0.0 | 20.0 | 0.9 | 10.0 | 1206.3 | 1231.1 | 17,970.3 | 21,906.7 | 1877.7 | 1879.5 | 2021.5 | 1983.9 | ||||||||||||
| 16 | 100.0 | 20.0 | 0.9 | 2.0 | 996.3 | 982.8 | 11,382.8 | 8691.5 | 1954.2 | 1955.3 | 2214.8 | 2212.0 | ||||||||||||
| 17 | 0.0 | 100.0 | 0.9 | 2.0 | 1197.1 | 1193.9 | 19,236.4 | 21,494.5 | 1918.9 | 1879.5 | 2006.9 | 1992.9 | ||||||||||||
| 18 | 100.0 | 100.0 | 0.9 | 10.0 | 1096.2 | 1058.8 | 17,911.0 | 18,259.6 | 1955.3 | 1955.3 | 2235.2 | 2235.6 | ||||||||||||
| 19 | 50.0 | 60.0 | 0.5 | 6.0 | 1350.7 | 1362.1 | 39,810.1 | 38,700.3 | 1833.3 | 1818.6 | 1665.0 | 1658.5 | ||||||||||||
| 20 | 50.0 | 60.0 | 0.5 | 6.0 | 1336.5 | 1362.1 | 46,239.4 | 38,700.3 | 1833.3 | 1818.6 | 1665.0 | 1658.5 | ||||||||||||
| 21 | 0.0 | 60.0 | 0.5 | 6.0 | 1264.4 | 1263.1 | 18,405.5 | 13,844.0 | 1896.6 | 1928.6 | 2153.1 | 2146.6 | ||||||||||||
| 22 | 100.0 | 60.0 | 0.5 | 6.0 | 783.1 | 789.6 | 6640.4 | 5619.0 | 1797.1 | 1735.5 | 1943.4 | 1861.0 | ||||||||||||
| 23 | 50.0 | 20.0 | 0.5 | 6.0 | 1254.6 | 1264.7 | 30,314.5 | 31,811.9 | 1807.3 | 1832.0 | 1823.5 | 1737.6 | ||||||||||||
| 24 | 50.0 | 100.0 | 0.5 | 6.0 | 1288.9 | 1284.0 | 33,835.8 | 36,389.9 | 1807.3 | 1832.0 | 1688.1 | 1705.2 | ||||||||||||
| 25 | 50.0 | 60.0 | 0.1 | 6.0 | 761.6 | 779.4 | 34,785.4 | 30,843.7 | 1793.4 | 1733.2 | 1586.1 | 1556.3 | ||||||||||||
| 26 | 50.0 | 60.0 | 0.9 | 6.0 | 1175.4 | 1162.7 | 35,161.2 | 37,358.2 | 1893.2 | 1930.8 | 1834.5 | 1886.5 | ||||||||||||
| 27 | 50.0 | 60.0 | 0.5 | 2.0 | 1175.0 | 1167.8 | 31,125.6 | 31,605.9 | 1786.5 | 1832.0 | 1691.2 | 1709.4 | ||||||||||||
| 28 | 50.0 | 60.0 | 0.5 | 10 | 1294.6 | 1304.8 | 34,152.3 | 36,596 | 1887.5 | 1832.0 | 1721.8 | 1733.4 | ||||||||||||
| 29 | 50.0 | 60.0 | 0.5 | 6 | 1245.5 | 1236.3 | 34,112.8 | 34,100.9 | 1825.6 | 1832.0 | 1657.3 | 1721.4 | ||||||||||||
| 30 | 50.0 | 60.0 | 0.5 | 6 | 1245.5 | 1236.3 | 33,736.9 | 34,100.9 | 1825.6 | 1832.0 | 1679.7 | 1721.4 | ||||||||||||

| Independent Variables | Regression Coefficient | |||
|---|---|---|---|---|
| Dependent Variables | ||||
| TPC | TFC | ABTS Radical Scavenging | DPPH Radical Scavenging | |
| (mg GAE/100 g dry weight, DW) | (mg CE/100 g dry weight, DW) | (µmol TEAC/100 g dry weight, DW) | (µmol TEAC/100 g dry weight, DW) | |
| Intercept, X0 | 1286.2 | 36,835 | 1826 | 1702.5 |
| Linear | ||||
| X1, Ethanol concentration | −236.8 *** | −4112.5 *** | −96.6 *** | −142.8 *** |
| X2, Amplitude | 9.7 | 2289 * | - | −16.2 |
| X3, Duty Cycle | 191.67 *** | 3257.2 ** | 98.8 *** | 165.1 *** |
| X4, Extraction time | 68.5 *** | 2495.1 * | - | 12 |
| Quadratic | ||||
| X12 | −209.9 *** | −24,369.4 *** | - | 282.5 *** |
| X22 | 38.01 ** | - | - | - |
| X32 | −265.2 *** | - | - | - |
| X42 | - | - | - | - |
| Interaction | ||||
| X12 | −67.4 *** | - | - | 97.6 *** |
| X13 | 140.9 *** | - | 134.4 *** | 260.4 *** |
| X14 | - | - | - | 114.4 *** |
| X23 | - | - | - | −90.1 *** |
| X24 | - | - | - | - |
| X34 | 27.2 *** | - | - | −106 *** |
| Model | ||||
| F value | 675.6 | 39.4 | 161.6 | 118.9 |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Lack of fit | ||||
| F value | 7.8 | 2.9 | - | 8.7 |
| p value | 0.058 | 0.2096 | - | 0.0504 |
| Mean | 1024 | 22,213.4 | 1826 | 1871.9 |
| Standard deviation | 20.8 | 4249 | 36.1 | 50.3 |
| R2 | 0.9975 | 0.8994 | 0.9528 | 0.9859 |
| Adjusted R2 | 0.996 | 0.8766 | 0.9469 | 0.9776 |
| Coefficient of variation | 2 | 19.1 | 2 | 2.7 |
2.3. Effects of Process Variables on Antioxidant Activity, ABTS•+ Radical Cations and DPPH• Radical Scavenging Capacity

2.4. Optimal Conditions
| Dependent Responses | Independent Variables | Optimum Value | |||||
|---|---|---|---|---|---|---|---|
| Ethanol Concentration (%, v/v) | Amplitude (%) | Duty Cycle (W/s) | Extraction Time (min) | Experimental a | Predicted | p-value | |
| Total phenolic content (TPC) (mg GAE/100 g DW) | 15.2 | 58.5 | 0.7 | 8.3 | 1383.8 | 1373.7 | 0.134 |
| Total flavonoid content (TFC) (mg CE/100 g DW) | 45.9 | 100 | 0.9 | 10 | 45,029 | 45,003 | 0.938 |
| ABTS radical scavenging capacity (µmol TEAC/100 g DW) | 100 | 99.2 | 0.9 | 10 | 1961.3 | 1949.6 | 0.097 |
| DPPH radical scavenging capacity (µmol TEAC/100 g DW) | 0 | 20 | 0.9 | 2.4 | 2423.3 | 2375.1 | 0.055 |
| Combination of TPC, TFC, ABTS &DPPH | 54.1 | 20 | 0.9 | 10 | 1332.9 | 1334.4 | 0.882 |
| 39793 | 39,701.3 | 0.804 | |||||
| 1927.9 | 1942.2 | 0.099 | |||||
| 1892.9 | 1915 | 0.459 | |||||
2.5. Identification of Phenolics in Orthosiphon stamineus Extract by HPLC-MS
2.6. Scanning Electron Microscopy (SEM)
3. Experimental Section
3.1. Plant Material

3.2. Chemicals and Reagents
3.3. Ultrasound-Assisted Extraction (UAE) from Orthosiphon Stamineus
3.4. Experimental Design
3.5. Analysis for Total Phenolic Content
3.6. Analysis for Total Flavonoid Content
3.7. Determination of Antioxidant Activity—ABTS•+ Radical Cation
3.8. Determination of Antioxidant Activity—DPPH• Radical Scavenging Capacity

3.9. Identification of Phenolics in Orthosiphon Stamineus Extract by HPLC-MS
3.10. Scanning Electron Microscopy (SEM)
3.11. Statistical Analysis

3.12. Verification of the Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spigno, G.; de Faveri, D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007, 78, 793–801. [Google Scholar]
- Khamsah, S.M.; Akowah, G.; Zhari, I. Antioxidant activity and phenolic content of Orthosiphon Stamineus Benth from different geographical origin. J. Sustain. Sci. Manag. 2006, 1, 14–20. [Google Scholar]
- Akowuah, G.A.; Zhari, I.; Norhayati, I.; Sadikun, A.; Khamsah, S.M. Sinensetin, eupatorin, 3'-hydroxy-5,6,7,4'-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chem. 2004, 87, 559–566. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Mohd Adzahan, N.; Sarker, M.D.I.; Ganjloo, A. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules 2012, 17, 11748–11762. [Google Scholar] [CrossRef]
- Lee, M.H.; Lin, C.C. Comparison of techniques for extraction of isoflavones from the root of Radix Puerariae: Ultrasonic and pressurized solvent extractions. Food Chem. 2007, 105, 223–228. [Google Scholar]
- Priego-Capote, F.; Luque de Castro, M.D. Analytical uses of ultrasound I. Sample preparation. Trend. Anal. Chem. 2004, 9, 644–653. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. 2008, 9, 161–169. [Google Scholar]
- Fan, G.; Han, Y.; Gu, Z.; Chen, D. Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM). LWT Food Sci. Technol. 2008, 41, 155–160. [Google Scholar]
- Gan, H.E.; Karim, R.; Muhammad, S.K.S.; Bakar, J.A.; Hashim, D.M.; Rahman, R.A. Optimization of the basic formulation of a traditional baked cassava cake using response surface methodology. Food Sci. Technol. 2007, 40, 611–618. [Google Scholar]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process And Product Optimization Using Designed Experiments; John Wiley & Sons, Inc: New York, NY, USA, 2002; p. 32. [Google Scholar]
- Jiaoyan, R.; Mouming, Z.; John, S.; Jinshui, W.; Yueming, J.; Chun, C.; Yukio, K.; Sophia, J.X. Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. LWT Food Sci. Technol. 2008, 41, 1624–1632. [Google Scholar]
- Zou, T.B.; Xia, E.Q.; He, T.P.; Huang, M.Y.; Jia, Q.; Li, H.W. Ultrasound-assisted Extraction of Mangiferin from Mango (Mangifera indica L.) leaves using response surface methodology. Molecules 2014, 19, 1411–1421. [Google Scholar] [CrossRef]
- Sivakumar, V.; Anna, J.L.; Vijayeeswarri, J.; Swaminathan, G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason. Sonochem. 2009, 16, 782–789. [Google Scholar] [CrossRef]
- Herrera, M.C.; Luque de Castro, M.D. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. J. Chromatogr. A 2005, 1100, 1–7. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Capelo, J.L.; Lavilla, I.; Bendicho, C. Comparison of ultrasound-assisted extraction and microwave-assisted digestion for determination of magnesium, manganese and zinc in plant samples by flame atomic absorption spectrometry. Talanta 2000, 53, 433–441. [Google Scholar]
- Paniwnyk, L.; Beaufoy, E.; Lorimer, J.P.; Mason, T.J. The extraction of rutin from flower buds of Sophora japonica. Ultrason. Sonochem. 2001, 8, 299–301. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar]
- Parejo, I.; Caprai, E.; Bastida, J.; Viladomat, F.; Jáuregui, O.; Codina, C. Investigation of Lepechinia graveolens for its antioxidant activity and phenolic composition. J. Ethnopharmacol. 2004, 94, 175–184. [Google Scholar] [CrossRef]
- Tolonen, A.; Uusitalo, J. Fast screening method for the analysis of total flavonoid content in plants and foodstuffs by high-performance liquid chromatography/electrospray ionization time-of-flight mass spectrometry with polarity switching. Rapid Commun. Mass Spectrom. 2004, 18, 3113–3122. [Google Scholar] [CrossRef]
- Wang, D.; Lu, J.; Miao, A.; Xie, Z.; Yang, D. HPLC-DAD-ESI-MS/MS analysis of polyphenols and purine alkaloids in leaves of 22 tea cultivars in China. J. Food Compos. Anal. 2008, 21, 361–369. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Aspé, E.; Fernández, K. The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata Bark. Ind. Crop. Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Chemat, S.; Lagha, A.; Ait Amar, H.; Bartels, P.V.; Chemat, F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavour Frag. J. 2004, 19, 188–195. [Google Scholar]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem. Pharmacol. 1998, 56, 213–222. [Google Scholar]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Cheng, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008, 110, 571–583. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.H.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar]
- Sample Availability: Samples of the extract are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ho, S.K.; Tan, C.P.; Thoo, Y.Y.; Abas, F.; Ho, C.W. Ultrasound-Assisted Extraction of Antioxidants in Misai Kucing (Orthosiphon stamineus). Molecules 2014, 19, 12640-12659. https://doi.org/10.3390/molecules190812640
Ho SK, Tan CP, Thoo YY, Abas F, Ho CW. Ultrasound-Assisted Extraction of Antioxidants in Misai Kucing (Orthosiphon stamineus). Molecules. 2014; 19(8):12640-12659. https://doi.org/10.3390/molecules190812640
Chicago/Turabian StyleHo, Swee Kheng, Chin Ping Tan, Yin Yin Thoo, Faridah Abas, and Chun Wai Ho. 2014. "Ultrasound-Assisted Extraction of Antioxidants in Misai Kucing (Orthosiphon stamineus)" Molecules 19, no. 8: 12640-12659. https://doi.org/10.3390/molecules190812640
APA StyleHo, S. K., Tan, C. P., Thoo, Y. Y., Abas, F., & Ho, C. W. (2014). Ultrasound-Assisted Extraction of Antioxidants in Misai Kucing (Orthosiphon stamineus). Molecules, 19(8), 12640-12659. https://doi.org/10.3390/molecules190812640