Biophysical Approach to Mechanisms of Cancer Prevention and Treatment with Green Tea Catechins
Abstract
:1. Introduction
2. Historical Development
3. Inhibition of Metastasis with EGCG and Green Tea Catechins
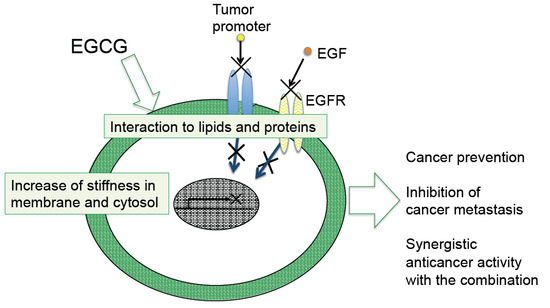
4. Sealing Effects of EGCG
5. Significance of Biophysical Phenotypes in Cancer Progression and Metastasis
6. Biophysical Effects with Green Tea Catechins
6.1. Increase in Stiffness of Cancer Cells with Green Tea Extract and EGCG
6.2. Other Biophysical Effects of Green Tea Catechins
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| DMBA | 7,12-dimethylbenz[a]anthracene |
| DMPC | Dimyristoylphosphatidylcholine |
| DNMT | DNA methyltransferase |
| DPPC | Dipalmitoylphosphatidylcholine |
| EC | (−)-Epicatechin |
| ECG | (−)-Epicatechin gallate |
| EGC | (−)-Epigallocatechin |
| EGCG | (−)-Epigallocatechin gallate |
| EMT | Epithelial-mesenchymal transition |
| Fucci | Fluorescent ubiquitination-based cell cycle/indicator |
| G.T.E | Green tea extract tablets |
| GTP | Green tea polyphenols |
| HDAC | Histone deacetylase |
| i.v. | Intravenous |
| MβCD | Methyl-β-cyclodextrin |
| PKC | Protein kinase C |
| PP1 | Protein phosphatase 1 |
| PP2A | Protein phosphatase 2A |
| RICM | Reflection interference contrast microscopy |
| SAM | Self-assembled monolayer |
| SAMP10 | Senescence-accelerated mice prone 10 |
| SCID | Severe immunodeficiency |
| SFCs | Sphere-forming cells |
| TGF-β | Transforming growth factor-β |
| Tipα | TNF-α inducing protein |
| TNF-α | Tumor necrosis factor-α |
| TPA | 12-O-Tetradecanoylphorbol-13-acetate |
| TSCC | Tongue squamous cell carcinoma |
References
- Fujiki, H. Green tea cancer prevention. In Encyclopedia of Cancer; Springer-Verlag: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Shimizu, M.; Fukutomi, Y.; Ninomiya, M.; Nagura, K.; Kato, T.; Araki, H.; Suganuma, M.; Fujiki, H.; Moriwaki, H. Green tea extracts for the prevention of metachronous colorectal adenomas: A pilot study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3020–3025. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.M. Chemoprevention in gastrointestinal cancers. In Proceedings of the International Conference on the 19th Annual Meeting of Korean Society of Cancer Prevention, Seoul, Korea, 12–13 December 2014.
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Horiuchi, T.; Fujiki, H.; Yoshida, T.; Okuda, T.; Sugimura, T. Antitumor promoting activity of (−)-epigallocatechin gallate, the main constituent of “tannin” in green tea. Phytother. Res. 1987, 1, 44–47. [Google Scholar] [CrossRef]
- Fujiki, H.; Imai, K.; Nakachi, K.; Shimizu, M.; Moriwaki, H.; Suganuma, M. Challenging the effectiveness of green tea in primary and tertiary cancer prevention. J. Cancer Res. Clin. Oncol. 2012, 138, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Saha, A.; Fujiki, H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011, 102, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Nanomedicine: Elastic clues in cancer detection. Nat. Nanotechnol. 2007, 2, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Horiuchi, T.; Suganuma, M.; Nishiwaki, S.; Yatsunami, J.; Okabe, S.; Okuda, T.; Muto, Y.; Frenkel, K.; Troll, W.; et al. Penta-O-galloyl-β-d-glucose and (−)-epigallocatechin gallate cancer preventive agents. In Phenolic Compounds in Food and their Prevention, Acs Symposium Series; Huang, M.T., Ho, C.T., Lee, C.Y., Eds.; American Chemical Society: Washington, DC, USA, 1992; Volume 507, pp. 316–325. [Google Scholar]
- Fujiki, H. Green tea: Health benefits as cancer preventive for humans. Chem. Rec. 2005, 5, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Okuda, T. (−)-epigallocatechin gallate. Drugs Future 1992, 17, 462–464. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, M.; Okabe, S.; Kai, Y.; Sueoka, N.; Sueoka, E.; Fujiki, H. Synergistic effects of (−)-epigallocatechin gallate with (−)-epicatechin, sulindac or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999, 59, 44–47. [Google Scholar] [PubMed]
- Fujiki, H.; Suganuma, M.; Okabe, S.; Sueoka, E.; Suga, K.; Imai, K.; Nakachi, K. A new concept of tumor promotion by tumor necrosis factor-α, and cancer preventive agents (−)-epigallocatechin gallate and green tea—A review. Cancer Detect. Prev. 2000, 24, 91–99. [Google Scholar] [PubMed]
- Suganuma, M.; Okabe, S.; Marino, M.W.; Sakai, A.; Sueoka, E.; Fujiki, H. Essential role of tumor necrosis factor α (TNF-α) in tumor promotion as revealed by TNF-α-deficient mice. Cancer Res. 1999, 59, 4516–4518. [Google Scholar] [PubMed]
- Nakachi, K.; Matsuyama, S.; Miyake, S.; Suganuma, M.; Imai, K. Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. Biofactors 2000, 13, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Imai, K.; Nakachi, K. Green tea: Cancer preventive beverage and/or drug. Cancer Lett. 2002, 188, 9–13. [Google Scholar] [CrossRef]
- Suganuma, M.; Kurusu, M.; Suzuki, K.; Tasaki, E.; Fujiki, H. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of GADD153 gene. Int J. Cancer 2006, 119, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. Oncol. 2015, 141, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Stearns, M.E.; Wang, M. Synergistic effects of the green tea extract epigallocatechin-3-gallate and taxane in eradication of malignant human prostate tumors. Transl. Oncol. 2011, 4, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Fujiki, H.; Kobayashi, H.; Go, H.; Miyado, K.; Sadano, H.; Shimokawa, R. Effect of (−)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992, 65, 51–54. [Google Scholar] [CrossRef]
- Liu, J.D.; Chen, S.H.; Lin, C.L.; Tsai, S.H.; Liang, Y.C. Inhibition of melanoma growth and metastasis by combination with (−)-epigallocatechin-3-gallate and dacarbazine in mice. J. Cell Biochem. 2001, 83, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Meleth, S.; Katiyar, S.K. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clin. Cancer Res. 2005, 11, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Amankwah, E.; Connors, S.; Park, H.Y.; Rincon, M.; Cornnell, H.; Chornokur, G.; Hashim, A.I.; Choi, J.; Tsai, Y.Y.; et al. Safety and chemopreventive effect of polyphenon E in preventing early and metastatic progression of prostate cancer in TRAMP mice. Cancer Prev. Res. 2014, 7, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kinouchi Shimizu, N.; Hakamata, W.; Unno, K.; Asai, T.; Oku, N. Preventive effect of green tea catechins on experimental tumor metastasis in senescence-accelerated mice. Biol. Pharm. Bull. 2010, 33, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Nakachi, K.; Suemasu, K.; Suga, K.; Takeo, T.; Imai, K.; Higashi, Y. Influence of drinking green tea on breast cancer malignancy among japanese patients. Jpn. J. Cancer Res. 1998, 89, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Suganuma, M.; Hayashi, M.; Sueoka, E.; Komori, A.; Fujiki, H. Mechanisms of growth inhibition of human lung cancer cell line, PC-9, by tea polyphenols. Jpn. J. Cancer Res. 1997, 88, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Ochiai, Y.; Aida, M.; Park, K.; Kim, S.J.; Nomura, T.; Suganuma, M.; Fujiki, H. Mechanistic aspects of green tea as a cancer preventive: Effect of components on human stomach cancer cell lines. Jpn. J. Cancer Res. 1999, 90, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Okabe, S.; Sueoka, E.; Suga, K.; Imai, K.; Nakachi, K.; Kimura, S. Mechanistic findings of green tea as cancer preventive for humans. Proc. Soc. Exp. Biol. Med. 1999, 220, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Okabe, S.; Sueoka, E.; Sueoka, N.; Fujimoto, N.; Goto, Y.; Matsuyama, S.; Imai, K.; Nakachi, K. Cancer prevention with green tea and monitoring by a new biomarker, hnRNP B1. Mutat. Res. 2001, 480–481, 299–304. [Google Scholar] [CrossRef]
- Masuda, M.; Wakasaki, T.; Toh, S.; Shimizu, M.; Adachi, S. Chemoprevention of head and neck cancer by green tea extract: EGCG-the role of EGFR signaling and “lipid raft”. J. Oncol. 2011, 2011, 540148. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Cancer and metastasis: Prevention and treatment by green tea. Cancer Metastasis Rev. 2010, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Watanabe, T.; Mondal, A.; Suzuki, K.; Kurusu-Kanno, M.; Li, Z.; Yamazaki, T.; Fujiki, H.; Suganuma, M. Mechanism-based inhibition of cancer metastasis with (−)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2014, 443, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.N.; Fu, J.; Nall, D.; Rodova, M.; Shankar, S.; Srivastava, R.K. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int. J. Cancer 2012, 131, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Nam, H.J.; Kang, H.J.; Kwon, H.W.; Lim, Y.C. Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of notch pathway. Eur. J. Cancer 2013, 49, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Mineva, N.D.; Paulson, K.E.; Naber, S.P.; Yee, A.S.; Sonenshein, G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS ONE 2013, 8, e73464. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Tsukamoto, S.; Huang, Y.; Makio, A.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci. Rep. 2016, 6, 19225. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bian, S.; Yang, C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis 2011, 32, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Chen, P.N.; Peng, C.Y.; Yu, C.H.; Chou, M.Y. Suppression of miR-204 enables oral squamous cell carcinomas to promote cancer stemness, EMT traits, and lymph node metastasis. Oncotarget 2016, 7, 20180–20192. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Nagao, T.; Ingolfsson, H.I.; Maxfield, F.R.; Andersen, O.S.; Kopelovich, L.; Weinstein, I.B. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007, 67, 6493–6501. [Google Scholar] [CrossRef] [PubMed]
- Kitano, K.; Nam, K.Y.; Kimura, S.; Fujiki, H.; Imanishi, Y. Sealing effects of (−)-epigallocatechin gallate on protein kinase C and protein phosphatase 2A. Biophys. Chem. 1997, 65, 157–164. [Google Scholar] [CrossRef]
- Kuzuhara, T.; Suganuma, M.; Fujiki, H. Green tea catechin as a chemical chaperone in cancer prevention. Cancer Lett. 2008, 261, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M. Discrimination between normal and cancerous cells using afm. Bionanoscience 2016, 6, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Kreth, J.; Zhu, L.; Sullivan, R.; Shi, W.; Qi, F.; Gimzewski, J.K. Nanomechanical properties of glucans and associated cell-surface adhesion of streptococcus mutans probed by atomic force microscopy under in situ conditions. Microbiology 2007, 153, 3124–3132. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Lee, G.Y.; Ong, C.N.; Lim, C.T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Lin, H.K.; Lin, I.H.; Chiou, Y.W.; Chen, H.W.; Liu, C.Y.; Harn, H.I.; Chiu, W.T.; Wang, Y.K.; Shen, M.R.; et al. Mechanical phenotype of cancer cells: Cell softening and loss of stiffness sensing. Oncotarget 2015, 6, 20946–20958. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, Y.; Ye, T.; Wang, D.; Mao, B. Discrimination between cervical cancer cells and normal cervical cells based on longitudinal elasticity using atomic force microscopy. Nanoscale Res. Lett. 2015, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M.; Laidler, P.; Gil, D.; Lekki, J.; Stachura, Z.; Hrynkiewicz, A.Z. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur. Biophys. J. 1999, 28, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, T.B.; Bao, C.H.; Chen, X.Y.; Wang, Y.; Wang, Q. Physical properties of gastrointestinal stromal tumors based on atomic force microscope analysis. Genet. Mol. Res. 2013, 12, 5774–5785. [Google Scholar] [CrossRef] [PubMed]
- Remmerbach, T.W.; Wottawah, F.; Dietrich, J.; Lincoln, B.; Wittekind, C.; Guck, J. Oral cancer diagnosis by mechanical phenotyping. Cancer Res. 2009, 69, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kuramochi, H.; Takahashi, A.; Imai, K.; Katsuta, N.; Nakayama, T.; Fujiki, H.; Suganuma, M. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (−)-epigallocatechin gallate-treated cells. J. Cancer Res. Clin. Oncol. 2012, 138, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Gostek, J.; Prauzner-Bechcicki, S.; Nimmervoll, B.; Mayr, K.; Pabijan, J.; Hinterdorfer, P.; Chtcheglova, L.A.; Lekka, M. Nano-characterization of two closely related melanoma cell lines with different metastatic potential. Eur. Biophys. J. 2015, 44, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, C.; Li, S.; Zhou, X.; Liu, Z.; He, Q.; Zhang, N.; Ngan, A.; Tang, B.; Wang, A. AFM nanoindentation detection of the elastic modulus of tongue squamous carcinoma cells with different metastatic potentials. Nanomedicine 2013, 9, 864–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, V.; Mythreye, K.; O'Brien, E.T.; Berchuck, A.; Blobe, G.C.; Superfine, R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011, 71, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Takahashi, A.; Suzuki, K.; Kurusu-Kanno, M.; Yamaguchi, K.; Fujiki, H.; Suganuma, M. Epithelial-mesenchymal transition in human gastric cancer cell lines induced by TNF-α-inducing protein of Helicobacter pylori. Int. J. Cancer 2014, 134, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Osborne, L.D.; Li, G.Z.; How, T.; O’Brien, E.T.; Blobe, G.C.; Superfine, R.; Mythreye, K. TGF-β regulates LARG and GEF-H1 during EMT to affect stiffening response to force and cell invasion. Mol. Biol. Cell. 2014, 25, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Luo, Q.; Liu, L.; Zhang, B.; Shi, Y.; Ju, Y.; Song, G. Biomechanical profile of cancer stem-like cells derived from MHCC97H cell lines. J. Biomech. 2016, 49, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sakaue-Sawano, A.; Kurokawa, H.; Morimura, T.; Hanyu, A.; Hama, H.; Osawa, H.; Kashiwagi, S.; Fukami, K.; Miyata, T.; Miyoshi, H.; et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008, 132, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Jin, Y.S.; Lu, Q.Y.; Rao, J.; Gimzewski, J.K. Green tea extract selectively targets nanomechanics of live metastatic cancer cells. Nanotechnology 2011, 22, 215101. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.G.; Rodrigueza, W.V.; Kilsdonk, E.P.; Stoudt, G.W.; Johnson, W.J.; Phillips, M.C.; Rothblat, G.H. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 1996, 271, 16026–16034. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, R.; Conte, V.; Escribano, J.; Elosegui-Artola, A.; Labernadie, A.; Valon, L.; Navajas, D.; Garcia-Aznar, J.M.; Munoz, J.J.; Roca-Cusachs, P.; et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 2016, 353, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Nagayama, M.; Tanaka, T.; Furusawa, M.; Kashimata, M.; Takeuchi, H. Membrane-rigidifying effects of anti-cancer dietary factors. Biofactors 2002, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Effects of green tea catechins on membrane fluidity. Pharmacology 1999, 59, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Margina, D.; Ilie, M.; Gradinaru, D. Quercetin and epigallocatechin gallate induce in vitro a dose-dependent stiffening and hyperpolarizing effect on the cell membrane of human mononuclear blood cells. Int. J. Mol. Sci. 2012, 13, 4839–4859. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nakachi, K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ 1995, 310, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Ito, K.; Masuda, K.; Kakinuma, E.; Sakamoto, R.; Iketaki, K.; Yamamoto, H.; Suganuma, M.; Kobayashi, N.; Nakabayashi, S.; et al. Quantitative evaluation of cancer cell adhesion to self-assembled monolayer-patterned substrates by reflection interference contrast microscopy. J. Phys. Chem. B 2016, 120, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.S.; Liu, D.; Martin, J.; Tang, X.M.; Lee, J.J.; El-Naggar, A.K.; Wistuba, I.; Culotta, K.S.; Mao, L.; Gillenwater, A.; et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev. Res. 2009, 2, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Hu, G.; Gu, D.; Ni, X. Having a promising efficacy on type II diabetes, it's definitely a green tea time. Curr. Med. Chem. 2015, 22, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kristen, A.V.; Lehrke, S.; Buss, S.; Mereles, D.; Steen, H.; Ehlermann, P.; Hardt, S.; Giannitsis, E.; Schreiner, R.; Haberkorn, U.; et al. Green tea halts progression of cardiac transthyretin amyloidosis: An observational report. Clin. Res. Cardiol. 2012, 101, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Mahler, A.; Mandel, S.; Lorenz, M.; Ruegg, U.; Wanker, E.E.; Boschmann, M.; Paul, F. Epigallocatechin-3-gallate: A useful, effective and safe clinical approach for targeted prevention and individualised treatment of neurological diseases? EPMA J. 2013, 4, 5. [Google Scholar] [CrossRef] [PubMed]






| Groups | Lymphogenous Metastasis with B16-BL6 Cells | Hematogenous Metastasis with B16-F10 Cells | ||
|---|---|---|---|---|
| Average Number of Lung Nodules | (% of Inhibition) | Average Number of Lung Nodules | (% of Inhibition) | |
| Control | 25 | >150 | ||
| 0.05% EGCG | 7 | 72% | 107 | >29% |
| 0.1% EGCG | 10 | 60% | 76 | >50% |
| Effects | Reference |
|---|---|
| (1) Inhibition of | |
| Receptor bindings of tumor promoters, hormones, and growth factors (Sealing effects) | [10,31] |
| Cancer cell growth of numerous cancer cell lines (in vitro and in vivo) | [11,13,18] |
| Invasion and migration | [13,18,34,35] |
| Angiogenesis | [18] |
| Inflammatory cytokines production, such as TNF-α | [11,13,18,31,32] |
| Proteasomal activity | [18] |
| Various enzyme activities, such as PKC, ODC, MAP kinases, TERT, and COX | [10,11,13,18] |
| Signaling pathways of EGFR, HGFR, and FGFR, | [33,42] |
| Epithelial-mesenchymal transition (EMT) | [34,35] |
| Spheroid formation of cancer stem cells | [36,37,38] |
| (2) Induction of | |
| Apoptosis | [11,13,18,29] |
| Cell cycle arrest at G0/G1 or G2/M | [11,13,18,30] |
| Phase II enzymes, such as GS | [18] |
| (3) Modification of | |
| Epigenetic regulation by affecting DNMT and HDAC | [18] |
| miRNA expression, such as miR210, let-7b, miR-1, miR-204 | [39,40,41] |
| Organs | Cancer Cells | Normal Cells | Methods | Ratio of Young‘s Moduli (Cancer/Normal) | Deformability (Cancer/Normal) | Reference |
|---|---|---|---|---|---|---|
| Breast | Metastatic cancer cells from breast cancer patients | Mesothelial cells in pleural fluids | AFM | 0.33 | [46] | |
| MCF-7 | MCF10 | AFM | 0.55–0.71 | [47] | ||
| MCF-7, MDA-MB 468 | M10 | AFM | 0.18–0.38 | [48] | ||
| MCF-7, MDA-MB-231 | MCF10 | Microfluidic optical stretcher | 2.0–3.2 | [49] | ||
| Cervix | Caski | CRK2614 | AFM | 0.33 | [50] | |
| SiHa, HeLa | Primary epithelial cells | AFM | 0.24–0.41 | [48] | ||
| Ovary | HEY A8, HEY | IOSE | AFM | 0.20–0.36 | [51] | |
| Bladder | Hu456, T24, BC3726 | Hu609, HCV29 | AFM | 0.08 0.03–0.14 | [52] | |
| TSGH8301, J82 | SVHUC-1 | AFM | 0.35–0.41 | [48] | ||
| Pancreas | Metastatic cancer cells from pancreatic cancer patient | Mesothelial cells in pleural fluids | AFM | 0.33 | [46] | |
| BxPC-3, PANC-1, ASPC-1, Mia-PaCa-2 | HPDE | 0.53–0.92 | [48] | |||
| Stomach | GIST cells from patients | Normal stomach cells | AFM | 0.53 | [53] | |
| Lung | Metastatic cancer cells from lung cancer patients | Mesothelial cells in pleural fluids | AFM | 0.33 | [46] | |
| Oral cavity | Oral cancer cells from patients | Epithelial cells from healthy donors | Microfluidic optical stretcher | 3.5 | [54] |
| High Metastatic Cancer Cells | Low Metastatic Cancer Cells | Ration of Young’s Moduli/Deformability (High Metastatic /Low Metastatic) | Correlate with | Methods | Reference |
|---|---|---|---|---|---|
| Melanoma | |||||
| B16-F10 | B16-F1 | 0.48 | migration and metastatic potential | AFM | [55] |
| WM226-4 (derived from metastatic tissue) | WM115 (derived from primary tumor) | 0.72 | AFM | [56] | |
| Ovary | |||||
| HEY A8 | HEY | 0.56 | migration and invasion potential | AFM | [51] |
| HEY | IGROV | 10 times * | migration and invasion potential | Magnetic tweezer system | [58] |
| Tongue squamous cell carcinoma | |||||
| Primary cancer cells from patients with metastasis | Primary cancer cells from patients without metastasis | 0.53 | migartion and invasion potential high vimentin and low E-cadherin expressions | AFM | [57] |
| Hepatoma | |||||
| Sphere-forming cells derived from MHCC97H | MHCC97H | 0.8 | migration potential Oct3/4 and CD133 expressions | AFM | [61] |
| Cells | Green Tea Extract or Catechins | Young’s Moduli (kPa) (before → after Treatment) | Fold Increase | Mechanisms | Reference |
|---|---|---|---|---|---|
| Tumor cells in pleural effusion from pancreatic (1); lung (3); ovarian (4); and breast (1) cancer patients | Green tea extract | 0.43 * → 2.53 * (0.2–0.6) (1.5–3.5) | 6.2 | [63] | |
| Normal mesothelial cells in pleural effusion | Green tea extract | 2.43 ** → 2.60 ** (1.7–2.9) (1.6–3.6) | 1.1 | [63] | |
| Lung cancer cells A549 | Green tea extract | 0.23 → 1.0 | 2.9 | Increase of F-actin | [63] |
| Mouse melanoma cells B16-F10 | EGCG | 0.44 → 0.80 | 1.8 | Alteration of membrane organization | [55] |
| EC | 0.44 → 0.36 | 0.8 | [55] | ||
| Lung cancer cells H1299 | EGCG | 1.24 → 2.55 | 1.8 | Alteration of membrane organization Inhibition of EMT | [35] |
| Lung cancer cells Lu99 | EGCG | 1.29 → 2.28 | 1.8 | Alteration of membrane organization Inhibition of EMT | [35] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suganuma, M.; Takahashi, A.; Watanabe, T.; Iida, K.; Matsuzaki, T.; Yoshikawa, H.Y.; Fujiki, H. Biophysical Approach to Mechanisms of Cancer Prevention and Treatment with Green Tea Catechins. Molecules 2016, 21, 1566. https://doi.org/10.3390/molecules21111566
Suganuma M, Takahashi A, Watanabe T, Iida K, Matsuzaki T, Yoshikawa HY, Fujiki H. Biophysical Approach to Mechanisms of Cancer Prevention and Treatment with Green Tea Catechins. Molecules. 2016; 21(11):1566. https://doi.org/10.3390/molecules21111566
Chicago/Turabian StyleSuganuma, Masami, Atsushi Takahashi, Tatsuro Watanabe, Keisuke Iida, Takahisa Matsuzaki, Hiroshi Y. Yoshikawa, and Hirota Fujiki. 2016. "Biophysical Approach to Mechanisms of Cancer Prevention and Treatment with Green Tea Catechins" Molecules 21, no. 11: 1566. https://doi.org/10.3390/molecules21111566
APA StyleSuganuma, M., Takahashi, A., Watanabe, T., Iida, K., Matsuzaki, T., Yoshikawa, H. Y., & Fujiki, H. (2016). Biophysical Approach to Mechanisms of Cancer Prevention and Treatment with Green Tea Catechins. Molecules, 21(11), 1566. https://doi.org/10.3390/molecules21111566