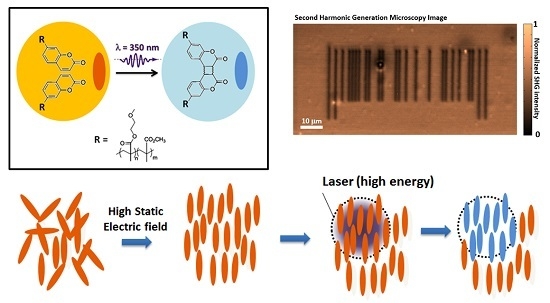
Coumarin-Containing Polymers for High Density Non-Linear Optical Data Storage
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis and Characterization of Copolymers P1–P21

| Polymer | R1 | R2 | (n/m)0 a | (n/m)obs b | Mn c | Mw c | Mv c | Ipd c | Tg d |
|---|---|---|---|---|---|---|---|---|---|
| P1 e | H | - | - | - | - | - | - | 120 | |
| P2 | H | CH3 | 1:1 | 1:1.4 | 14,700 | 28,100 | 25,600 | 1.9 | 112 |
| P3 | H | CH3 | 1:3 | 1:2.95 | 14,500 | 24,800 | 22,900 | 1.7 | 88 |
| P4 | H | CH3 | 1:5 | 1:5.57 | 21,900 | 34,900 | 32,500 | 1.6 | 83 |
| P5 | H | (CH2)3CH3 | 1:1 | 1:1.16 | 15,900 | 31,400 | 28,100 | 1.9 | 85 |
| P6 | H | (CH2)3CH3 | 1:3 | 1:3.9 | 27,200 | 52,100 | 47,000 | 1.9 | 73 |
| P7 e | CH3 | - | - | - | - | - | - | 152 | |
| P8 | CH3 | CH3 | 1:1 | 1:1.18 | 21,200 | 34,000 | 31,000 | 1.6 | 143 |
| P9 | CH3 | CH3 | 1:3 | 1:3 | 18,900 | 33,000 | 30,400 | 1.7 | 110 |
| P10 | CH3 | CH3 | 1:5 | 1:5.52 | 20,500 | 33,200 | 30,800 | 1.6 | 103 |
| P11 | CH3 | (CH2)3CH3 | 1:1 | 1:1.3 | 18,600 | 36,000 | 32,800 | 1.9 | 81 |
| P12 | CH3 | (CH2)3CH3 | 1:3 | 1:3 | 17,000 | 32,100 | 29,300 | 1.9 | 76 |
| Polymer | R1 | x | R2 | (n/m)0 a | (n/m)obs b | Mn c | Mw c | Mv c | Ipd c | Tg d |
|---|---|---|---|---|---|---|---|---|---|---|
| P13 | CH3 | 1 | CH3 | 1:3 | 1:4 | - | 16,900 | 16,900 | 1.4 | 90 |
| P14 | CH3 | 5 | CH3 | 1:1 | 1:1.32 | 13,600 | 25,000 | 23,000 | 1.8 | 40 |
| P15 | CH3 | 5 | CH3 | 1:3 | 1:3.21 | 17,900 | 36,600 | 24,000 | 2 | 47 |
| P16 | CH3 | 1 | CH3 | 1:1 | 1:1.11 | 6000 | 11,700 | 10,100 | 2 | 85 |
| P17 | CH3 | 5 | CH3 | 1:5 | 1:6 | 16,500 | 29,700 | 24,700 | 1.8 | 80 |
| P18 | H | 1 | CH3 | 1:3 | 1:2.56 | 13,000 | 27,000 | 14,700 | 2.0 | 93 |
| P19 | H | 1 | CH3 | 1:1 | 1:1.2 | 9700 | 17,200 | 18,900 | 1.7 | 81 |
| P20 | H | 5 | CH3 | 1:3 | 1:3 | 16,000 | 30,400 | - | 1.9 | 73 |
| P21 | H | 5 | CH3 | 1:1 | 1:1.75 | 12,000 | 23,500 | 21,000 | 1.95 | 50 |
2.2. UV-Vis Spectroscopy

2.3. Second Order Nonlinear Response
2.4. Comparative Investigation of the Optical Data Recording Efficiency
| Polymer | SHG bit 1 (arb. units) | SHG bit 0 (arb. units) | Normalized SHG bit 1 | Normalized SHG bit 0 | Contrast (%) |
|---|---|---|---|---|---|
| P9 | 3.38 | 3.44 | 0.983 | 1 | 0.88 |
| P13 | 3.23 | 3.34 | 0.967 | 1 | 1.67 |
| P3 | 3.24 | 3.33 | 0.973 | 1 | 1.37 |
| P18 | 4.2 | 8.75 | 0.480 | 1 | 35.14 |
| P2 | 3.23 | 3.35 | 0.964 | 1 | 1.82 |
| P8 | 2.33 | 2.41 | 0.967 | 1 | 1.69 |

2.5. Image Storage

3. Experimental Section
3.1. Characterization Techniques
3.2. Thin Films Preparation
3.3. Monomer Synthesis
3.3.1. 7-(2-Hydroxyethoxy)coumarin (1b)
3.3.2. 7-[(6-Hydroxyhexyl)oxy]coumarin (1c)
3.3.3. 7-(2-Hydroxyethoxy)4-methylcoumarin (2b)
3.3.4. 7-[(6-Hydroxyhexyl)oxy]-4-methylcoumarin (2c)
3.3.5. 7-Methacryloyloxycoumarin (3a)
3.3.6. 7-(2-Methacryloyloxyethoxy)coumarin (3b)
3.3.7. 7-[6-Methacryloyloxyhexyloxy]coumarin (3c)
3.3.8. 7-Methacryloyloxy-4-methylcoumarin (4a)
3.3.9. 7-(2-Methacryloyloxyethoxy)4-methylcoumarin (4b)
3.3.10. 7-[[[6-(Methacryloyl)oxy]hexyl]oxy]-4-methylcoumarin (4c)
3.4. Polymerization
3.5. Molecular Orientation in Thin Films by Corona Poling

3.6. Non-Linear Microscopy Setup

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Church, G.M.; Gao, Y.; Kosuri, S. Next-generation digital information storage in DNA. Science 2012, 337, 1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gecevičius, M.; Beresna, M.; Kazansky, P.G. Seemingly unlimited lifetime data storage in nanostructured glass. Phys. Rev. Lett. 2014, 112, 33901. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, P.; Chon, J.W.M.; Gu, M. Five-dimensional optical recording mediated by surface plasmons in gold nanorods. Nature 2009, 459, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Parthenopoulos, D.A.; Rentzepis, P.M. Three-dimensional optical storage memory. Science 1989, 245, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Dvornikov, A.S.; Walker, E.P.; Rentzepis, P.M. Two-photon three-dimensional optical storage memory. J. Phys. Chem. A 2009, 113, 13633–13644. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Sun, L.; Tang, H.; Wen, Y.; Jiang, G.; Huang, W.; Jiang, L.; Song, Y.; Tian, H.; Zhu, D. A novel thermally stable spironaphthoxazine and its application in rewritable high density optical data storage. Adv. Mater. 2005, 17, 156–160. [Google Scholar] [CrossRef]
- Corredor, C.C.; Huang, Z.-L.; Belfield, K.D. Two-photon 3D optical data storage via fluorescence modulation of an efficient fluorene dye by a photochromic diarylethene. Adv. Mater. 2006, 18, 2910–2914. [Google Scholar] [CrossRef]
- Lott, J.; Ryan, C.; Valle, B.; Johnson, J.R.; Schiraldi, D.A.; Shan, J.; Singer, K.D.; Weder, C. Two-photon 3D optical data storage via aggregate switching of excimer-forming dyes. Adv. Mater. 2011, 23, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Kawata, S.; Kawata, Y. Three-dimensional optical data storage using photochromic materials. Chem. Rev. 2000, 100, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Gindre, D.; Boeglin, A.; Fort, A.; Mager, L.; Dorkenoo, K.D. Rewritable optical data storage in azobenzene copolymers. Opt. Express 2006, 14, 9896–9901. [Google Scholar] [CrossRef] [PubMed]
- Gindre, D.; Boeglin, A.; Taupier, G.; Crégut, O.; Vola, J.; Barsella, A.; Mager, L.; Fort, A.; Dorkenoo, K.D. Toward submicrometer optical storage through controlled molecular disorder in azo-dye copolymer films. J. Opt. Soc. Am. B 2007, 24, 532–537. [Google Scholar] [CrossRef]
- Spiridon, M.C.; Iliopoulos, K.; Jerca, F.A.; Jerca, V.V.; Vuluga, D.M.; Vasilescu, D.S.; Gindre, D.; Sahraoui, B. Novel pendant azobenzene/polymer systems for second harmonic generation and optical data storage. Dye. Pigment 2015, 114, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chon, J.W.M.; Evans, R.A.; Gu, M. Two-photon energy transfer enhanced three-dimensional optical memory in quantum-dot and azo-dye doped polymers. Appl. Phys. Lett. 2008, 92, 063309. [Google Scholar] [CrossRef]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in polymers: From light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev. 2004, 104, 3059–3077. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, L.; Krupka, O.; Smokal, V.; Grabowski, A.; Naparty, M.; Derkowska-Zielinska, B. Optical properties of coumarins containing copolymers. Opt. Mater. 2015, 47, 18–23. [Google Scholar] [CrossRef]
- Belfield, K.D.; Bondar, M.V.; Liu, Y.; Przhonska, O.V. Photophysical and photochemical properties of 5,7-dimethoxycoumarin under one- and two-photon excitation. J. Phys. Org. Chem. 2003, 16, 69–78. [Google Scholar] [CrossRef]
- Gnanaguru, K.; Ramasubbu, N.; Venkatesan, K.; Ramamurthy, V. A study on the photochemical dimerization of coumarins in the solid state. J. Org. Chem. 1985, 50, 2337–2346. [Google Scholar] [CrossRef]
- Sato, E.; Nagai, S.; Matsumoto, A. Reversible thickness control of polymer thin films containing photoreactive coumarin derivative units. Prog. Org. Coatings 2013, 76, 1747–1751. [Google Scholar] [CrossRef]
- Härtner, S.; Kim, H.-C.; Hampp, N. Photodimerized 7-hydroxycoumarin with improved solubility in PMMA: Single-photon and two-photon-induced photocleavage in solution and PMMA films. J. Photochem. Photobiol. A Chem. 2007, 187, 242–246. [Google Scholar] [CrossRef]
- Wolff, T.; Görner, H. Photocleavage of dimers of coumarin and 6-alkylcoumarins. J. Photochem. Photobiol. A Chem. 2010, 209, 219–223. [Google Scholar] [CrossRef]
- Buckup, T.; Dorn, J.; Hauer, J.; Härtner, S.; Hampp, N.; Motzkus, M. The photoinduced cleavage of coumarin dimers studied with femtosecond and nanosecond two-photon excitation. Chem. Phys. Lett. 2007, 439, 308–312. [Google Scholar] [CrossRef]
- Iliopoulos, K.; Krupka, O.; Gindre, D.; Sallé, M. Reversible two-photon optical data storage in coumarin-based copolymers. J. Am. Chem. Soc. 2010, 132, 14343–14345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essaïdi, Z.; Krupka, O.; Iliopoulos, K.; Champigny, E.; Sahraoui, B.; Sallé, M.; Gindre, D. Synthesis and functionalization of coumarin-containing copolymers for second order optical nonlinearities. Opt. Mater. 2013, 35, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Gindre, D.; Iliopoulos, K.; Krupka, O.; Champigny, E.; Morille, Y.; Sallé, M. Image storage in coumarin-based copolymer thin films by photoinduced dimerization. Opt. Lett. 2013, 38, 4636–4639. [Google Scholar] [CrossRef] [PubMed]
- Gindre, D.; Sallé, M.; Krupka, O.; Iliopoulos, K. Reversible Recording Medium based On Optical Storage of Information, Method of Reversible Recording on Such a Medium. U.S. Patent 20,130,033,975 A1, 7 February 2013. [Google Scholar]
- Mortazavi, M.A.; Knoesen, A.; Kowel, S.T.; Higgins, B.G.; Dienes, A. Second-harmonic generation and absorption studies of polymer—dye films oriented by corona-onset poling at elevated temperatures. J. Opt. Soc. Am. B 1989, 6, 733–741. [Google Scholar] [CrossRef]
- Jackson, P.O.; O’Neill, M.; Duffy, W.L.; Hindmarsh, P.; Kelly, S.M.; Owen, G.J. An investigation of the role of cross-linking and photodegradation of side-chain coumarin polymers in the photoalignment of liquid crystals. Chem. Mater. 2001, 13, 694–703. [Google Scholar] [CrossRef]
- Tsutsumi, N.; Matsumoto, O.; Sakai, W.; Kiyotsukuri, T. Nonlinear optical polymers. 2. Novel NLO linear polyurethane with dipole moments aligned transverse to the main backbone. Macromolecules 1996, 29, 592–597. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds P1–P21 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gindre, D.; Iliopoulos, K.; Krupka, O.; Evrard, M.; Champigny, E.; Sallé, M. Coumarin-Containing Polymers for High Density Non-Linear Optical Data Storage. Molecules 2016, 21, 147. https://doi.org/10.3390/molecules21020147
Gindre D, Iliopoulos K, Krupka O, Evrard M, Champigny E, Sallé M. Coumarin-Containing Polymers for High Density Non-Linear Optical Data Storage. Molecules. 2016; 21(2):147. https://doi.org/10.3390/molecules21020147
Chicago/Turabian StyleGindre, Denis, Konstantinos Iliopoulos, Oksana Krupka, Marie Evrard, Emilie Champigny, and Marc Sallé. 2016. "Coumarin-Containing Polymers for High Density Non-Linear Optical Data Storage" Molecules 21, no. 2: 147. https://doi.org/10.3390/molecules21020147
APA StyleGindre, D., Iliopoulos, K., Krupka, O., Evrard, M., Champigny, E., & Sallé, M. (2016). Coumarin-Containing Polymers for High Density Non-Linear Optical Data Storage. Molecules, 21(2), 147. https://doi.org/10.3390/molecules21020147