Anti-Metastatic Properties of a Marine Bacterial Exopolysaccharide-Based Derivative Designed to Mimic Glycosaminoglycans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of OS-EPS Derivatives
2.2. In Vitro Effect of OS-EPS on Osteosarcoma Cell Lines
2.2.1. Cell Proliferation and Cell Viability
2.2.2. Cell Migration Assay
2.2.3. Cell Invasion Assay
2.2.4. Cell Cycle Analysis
2.2.5. Expression in Human HOS Osteosarcoma Cell Line of Matrix Metalloproteinases (MMPs) and Their Inhibitors (TIMPs)
2.3. In Vivo Studies
2.3.1. Primary Malignant Bone Tumor Growth
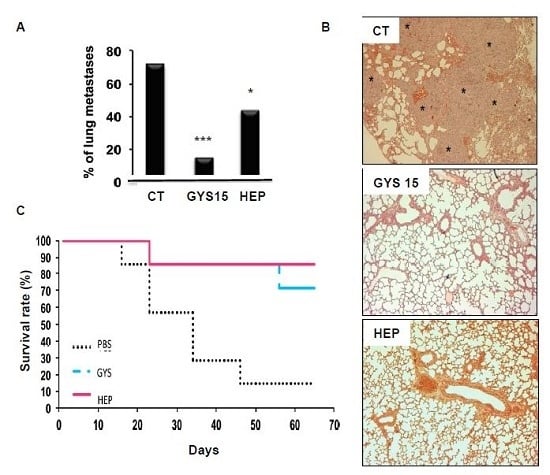
2.3.2. Model of Lung Metastases from Mouse Osteosarcoma
3. Experimental Section
3.1. General
3.2. Proliferation Assay
3.3. Migration Assay
3.4. Invasion Assay
3.5. Cell Cycle Analysis
3.6. Matrix Metalloproteinase Expression
3.7. Animal Ethics
3.8. Osteosarcoma Mouse Model
3.9. Lung Metastasis Mouse Model
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ando, K.; Mori, K.; Verrecchia, F.; Marc, B.H.; Redini, F.; Heymann, D. Molecular alterations associated with osteosarcoma development. Sarcoma 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odri, G.; Kim, P.-P.; Lamoureux, F.; Charrier, C.; Battaglia, S.; Amiaud, J.; Heymann, D.; Gouin, F.; Redini, F. Zoledronic acid inhibits pulmonary metastasis dissemination in a preclinical model of Ewing’s sarcoma via inhibition of cell migration. BMC Cancer 2014, 14, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkan, D.; Green, J.E.; Chambers, A.F. Extracellular matrix: A gatekeeper in the transition from dormancy to metastatic growth. Eur. J. Cancer 2010, 46, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.L.; Varki, A.; Borsig, L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb. Res. 2007, 120, S107–S111. [Google Scholar] [CrossRef]
- Velasco, C.R.; Colliec-Jouault, S.; Redini, F.; Heymann, D.; Padrines, M. Proteoglycans on bone tumor development. Drug Discov. Today 2010, 15, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Ferro, V.; Fewings, K.; Palermo, M.C.; Li, C.P. Large-scale preparation of the oligosaccharide phosphate fraction of Pichia holstii NRRL Y-2448 phosphomannan for use in the manufacture of PI-88. Carbohydr. Res. 2001, 332, 183–189. [Google Scholar] [CrossRef]
- Vismara, E.; Coletti, A.; Valerio, A.; Naggi, A.; Urso, E.; Torri, G. Anti-metastatic Semi-synthetic Sulfated Maltotriose C-C Linked Dimers. Synthesis and Characterisation. Molecules 2012, 17, 9912–9930. [Google Scholar] [PubMed]
- Feng, L.; Jia, X.-B.; Shi, F.; Chen, Y. Identification of Two Polysaccharides from Prunella vulgaris L. and Evaluation on Their Anti-Lung Adenocarcinoma Activity. Molecules 2010, 15, 5093–5103. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, P. Glycosaminoglycan polysaccharide biosynthesis and production: Today and tomorrow. Appl. Microbiol. Biotechnol. 2012, 94, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Colliec-Jouault, S.; Bavington, C.; Delbarre-Ladrat, C. Heparin-like Entities from Marine Organisms. Handb. Exp. Pharmacol. 2012, 207, 423–449. [Google Scholar] [PubMed]
- Colliec Jouault, S.; Chevolot, L.; Helley, D.; Ratiskol, J.; Bros, A.; Sinquin, C.; Roger, O.; Fischer, A.M. Characterization, chemical modifications and in vitro anticoagulant properties of an exopolysaccharide produced by Alteromonas infernus. Biochim. Biophys. Acta 2001, 1528, 141–151. [Google Scholar] [CrossRef]
- Roger, O.; Kervarec, N.; Ratiskol, J.; Colliec-Jouault, S.; Chevolot, L. Structural studies of the main exopolysaccharide produced by the deep-sea bacterium Alteromonas infernus. Carbohydr. Res. 2004, 339, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Velasco, C.; Baud’huin, M.; Sinquin, C.; Maillasson, M.; Heymann, D.; Colliec-Jouault, S.; Padrines, M. Effects of a sulfated exopolysaccharide produced by Altermonas infernus on bone biology. Glycobiology 2011, 21, 781–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laubli, H.; Borsig, L. Heparins attenuate cancer metastasis: Are selectins the link? Cancer Investig. 2009, 27, 474–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falanga, A.; Marchetti, M. Heparin in tumor progression and metastatic dissemination. Semin. Thromb. Hemost. 2007, 33, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Fritze, J.; Alban, S.; Ludwig, R.J.; Rubant, S.; Boehncke, W.H.; Schumacher, G.; Bendas, G. The influence of various structural parameters of semisynthetic sulfated polysaccharides on the P-selectin inhibitory capacity. Biochem. Pharmacol. 2006, 72, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Kapp, T.G.; Rechenmacher, F.; Sobahi, T.R.; Kessler, H. Integrin modulators: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1273–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, X.; Li, H.; Li, B.; Sun, L.; Xie, T.; Zhu, T.; Zhou, H.; Ye, Z. The role of Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharmacol. 2015, 24, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Liu, L.; Yang, W.J.; Zang, L.N.; Xi, Y.M. RNAi-mediated knockdown of relaxin decreases in vitro proliferation and invasiveness of osteosarcoma MG-63 cells by inhibition of MMP-9. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1102–1109. [Google Scholar] [PubMed]
- Li, H.; Zhang, K.; Liu, L.H.; Ouyang, Y.; Bu, J.; Guo, H.B.; Xiao, T. A systematic review of matrix metalloproteinase 9 as a biomarker of survival in patients with osteosarcoma. Tumour Biol. 2014, 5, 5487–5491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Liu, F.; Li, Z. Comments on Li H et al. “A systematic review of matrix metalloproteinase 9 as a biomarker of survival in patients with osteosarcoma”. Tumour Biol. 2015, 36, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Cottam, D.; Rees, R. Regulation of matrix metalloproteinases—Their role in tumor invasion and metastasis. Int. J. Oncol. 1993, 2, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Dou, C.; Jia, Y.; Tu, K.; Zheng, X. TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget 2015, 6, 12061–12079. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, S.; Ghoshal-Gupta, S.; Kutiyanawalla, A.; Gayatri, S.; Lee, B.R.; Jiwani, S.; Rojiani, A.M.; Rojiani, M.V. TIMPS-1 inhibits apotosis in lung adenocarcinoma cells via interaction with Bcl-2. PLoS ONE 2015, 10, e0137673. [Google Scholar] [CrossRef] [PubMed]
- Ory, B.; Heymann, M.F.; Kamijo, A.; Gouin, F.; Heymann, D.; Redini, F. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer 2005, 104, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Rojiani, M.V.; Ghoshal-Gupta, S.; Kutiyanawalla, A.; Mathur, S.; Rojiani, A.M. TIMP-1 overexpression in lung carcinoma enhances tumor kinetics and angiogenesis in brain metastasis. J. Neuropathol. Exp. Neurol. 2015, 74, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Seubert, B.; Grünwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N.; et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.G.; McDonnell, P.; Burke, M.B.; Deen, K.C.; Lyn, S.; Silverman, C.; Dul, E.; Appelbaum, E.R.; Eichman, C.; DiPrinzio, R.; et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 1998, 273, 14363–14367. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Croucher, P.I.; Hamdy, F.C.; Eaton, C.L. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002, 62, 1619–1623. [Google Scholar] [PubMed]
- Lamoureux, F.; Picarda, G.; Garrigue, L.; Baud’huin, M.; Trichet, V.; Vidal, A.; Miot-Noirault, E.; Pitard, B.; Heymann, D.; Redini, F. Glycosaminoglycans as potential regulators of osteoprotegerin therapeutic activity in osteosarcoma. Cancer Res. 2009, 69, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Guezennec, J.; Pignet, P.; Lijour, Y.; Gentric, E.; Ratiskol, J.; Colliec-Jouault, S. Sulfation and depolymerization of a bacterial exopolysaccharide of hydrothermal origin. Carbohydr. Polym. 1998, 37, 19–24. [Google Scholar] [CrossRef]
- Chopin, N.; Sinquin, C.; Ratiskol, J.; Zykwinska, A.; Weiss, P.; Cérantola, S.; Le Bideau, J.; Colliec-Jouault, S. A Direct Sulfation Process of a Marine Polysaccharide in Ionic Liquid. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Senni, K.; Gueniche, F.; Yousfi, M.; Fioretti, F.; Godeau, G.; Colliec-Jouault, S.; Ratiskol, J.; Sinquin, C.; Raguenes, G.; Courtois, A.; et al. Sulfated Depolymerized Derivatives of Exopolysaccharides (EPS) from Mesophilic Marine Bacteria, Method for Preparing Same, and Use Thereof in Tissue Regeneration. Patent WO 2006/003290, 4 May 2006. [Google Scholar]
- Ségaliny, A.I.; Mohamadi, A.; Dizier, B.; Lokajczyk, A.; Brion, R.; Lanel, R.; Amiaud, J.; Charrier, C.; Boisson-Vidal, C.; Heymann, D. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int. J. Cancer 2015, 137, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Moriceau, G.; Roelofs, A.J.; Brion, R.; Redini, F.; Ebetino, F.H.; Rogers, M.J.; Heymann, D. Synergistic inhibitory effect of apomine and lovastatin on osteosarcoma cell growth. Cancer 2012, 118, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Arpicco, S.; Milla, P.; Stella, B.; Dosio, F. Hyaluronic Acid Conjugates as Vectors for the Active Targeting of Drugs, Genes and Nanocomposites in Cancer Treatment. Molecules 2014, 19, 3193–3230. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds such as EPS and OS-EPS are available from the authors.







| EPS Derivatives | Mw * g/mol | Mn * g/mol | I * Mw/Mn | S ** % | Neutral Sugars *** % | Acidic Sugars **** % |
|---|---|---|---|---|---|---|
| OS EPS GYS15 | 16000 | 14000 | 1.14 | 15 | 18 | 10 |
| OS EPS GYS8 | 10000 | 8000 | 1.25 | 13 | 19 | 12 |
| OS EPS GYS4 | 5300 | 4700 | 1.13 | 14 | 23 | 11 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heymann, D.; Ruiz-Velasco, C.; Chesneau, J.; Ratiskol, J.; Sinquin, C.; Colliec-Jouault, S. Anti-Metastatic Properties of a Marine Bacterial Exopolysaccharide-Based Derivative Designed to Mimic Glycosaminoglycans. Molecules 2016, 21, 309. https://doi.org/10.3390/molecules21030309
Heymann D, Ruiz-Velasco C, Chesneau J, Ratiskol J, Sinquin C, Colliec-Jouault S. Anti-Metastatic Properties of a Marine Bacterial Exopolysaccharide-Based Derivative Designed to Mimic Glycosaminoglycans. Molecules. 2016; 21(3):309. https://doi.org/10.3390/molecules21030309
Chicago/Turabian StyleHeymann, Dominique, Carmen Ruiz-Velasco, Julie Chesneau, Jacqueline Ratiskol, Corinne Sinquin, and Sylvia Colliec-Jouault. 2016. "Anti-Metastatic Properties of a Marine Bacterial Exopolysaccharide-Based Derivative Designed to Mimic Glycosaminoglycans" Molecules 21, no. 3: 309. https://doi.org/10.3390/molecules21030309
APA StyleHeymann, D., Ruiz-Velasco, C., Chesneau, J., Ratiskol, J., Sinquin, C., & Colliec-Jouault, S. (2016). Anti-Metastatic Properties of a Marine Bacterial Exopolysaccharide-Based Derivative Designed to Mimic Glycosaminoglycans. Molecules, 21(3), 309. https://doi.org/10.3390/molecules21030309