Synthesis of Isoxazole and 1,2,3-Triazole Isoindole Derivatives via Silver- and Copper-Catalyzed 1,3-Dipolar Cycloaddition Reaction †
Abstract
:1. Introduction
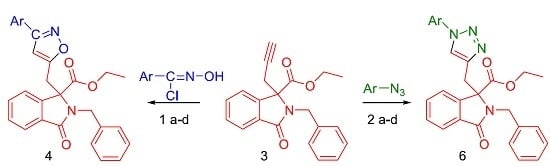
2. Results and Discussion
2.1. Spectroscopic and Crystallographic Characterization of Isoxazole 4a
2.2. Spectroscopic Characterization of Triazole 6a
3. Experimental Section
3.1. General Information
3.2. Typical Procedure of the Cycloaddition Reaction of Arylnitrile Oxides 1a–d and Ethyl-3-alkyldiynyl-phtalimidine-3-carboxylate (3)
3.3. Typical Procedure of the Cycloaddition Reaction of Azides 2a–d and Ethyl 3-Alkyldiynylphtalimidine-3-carboxylate (3)
3.4. X-ray Diffraction Structure Analysis of 4a
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Wannassi, N.; Rammah, M.M.; Boudriga, S.; Rammah, M.B.; Monnier-Jobé, K.; Ciamala, K.; Knorr, M.; Enescu, M.; Rousselin, Y.; Kubicki, M.M. Regio- and Stereoselective 1,3-Dipolar Cycloaddition of C-Aryl-N-phenylnitrones over (E)-Arylidene-(2H)-indan-1-ones: Synthesis of Highly Substituted Novel Spiro-isoxazolidines. Heterocycles 2010, 81, 2749–2762. [Google Scholar]
- Boudriga, S.; Wannassi, N.; Askri, M.; Rammah, M.E.B.; Strohmann, C. Diastereoselective synthesis and structure of spiroisoxaziline derivatives. J. Soc. Chim. Tunise 2009, 11, 29–36. [Google Scholar]
- Askri, M.; Rammah, M.; Monnier-Jobé, K.; Ciamala, K.; Knorr, M.; Strohmann, C. Synthesis of Some Spirochroman-4-Ones by Regioselective [4+2] Cycloaddition Reactions. Lett. Org. Chem. 2007, 4, 221–227. [Google Scholar] [CrossRef]
- Laouiti, A.; Rammah, M.; Askri, M.; Rammah, M.E.B. 1,3-dipolar cycloaddition of arylnitrile oxides with ethyl 1-allyl-2-benzyl-3-oxo-2,3-dihydro-1H-isoindole-1-carboxylates. J. Soc. Chim. Tunis. 2014, 16, 83–88. [Google Scholar]
- Padwa, A.; Pearson, W.H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; John Wiley and Sons: New York, NY, USA, 2003; Volume 59, pp. 1–940. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Padwa, A.; Huisgen, R. 1,3-Dipolar Cycloaddition Chemistry; John Wiley & Sons: New York, NY, USA, 1984; Volume 1. [Google Scholar]
- Huisgen, R.; Seidel, M.; Wallbilich, G.; Knupfer, H. Diphenylnitrilimine and its 1,3-dipolar additions to alkenes and alkynes. Tetrahedron Lett. 1962, 17, 3–29. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Huisgen, R. Neues über 1,3-Cycloadditionen. Helv. Chim. Acta 1967, 50, 2421–2439. [Google Scholar] [CrossRef]
- Huisgen, R.; Knupfer, H.; Sustmann, R.; Wallbilich, G. 1.3-Dipolare Cycloadditionen. Zur Anlagerung des Diphenylnitrilimins an nichtkonjugierte Alkene und Alkine; Sterischer Ablauf, Orientierung un Substituenteneinfluß. Chem. Ber. 1967, 100, 1580–1592. [Google Scholar] [CrossRef]
- Huisgen, R.; Knupfer, H.; Sustmann, R.; Wallbilich, G.; Clovis, J.S.; Eckel, A. 1,3-Dipolare Cycloadditionen. Diphenylnitrilimin und arylkonjugierte Alkene. Chem. Ber. 1967, 100, 1593–1601. [Google Scholar]
- Padwa, A. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 4, p. 1069. [Google Scholar]
- Gothelf, K.V.; Jørgensen, K.A. Asymmetric 1,3-Dipolar Cycloaddition Reactions. Chem. Rev. 1998, 98, 863–909. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Weingarten, M.D. Cascade Processes of Metallo Carbenoids. Chem. Rev. 1996, 96, 223–270. [Google Scholar] [CrossRef] [PubMed]
- Hauck, S.I.; Cederberg, C.; Doucette, A.; Grosser, L.; Hales, N.J.; Poon, G.; Gravestock, M.B. New carbon-linked azole oxazolidinones with improved potency and pharmacokinetics. Bioorg. Med. Chem. Lett. 2007, 17, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Malawska, B. New anticonvulsant agents. Curr. Top. Med. Chem. 2005, 5, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Mahalinga, M.; Karthikeyen, M.S.; Poojary, B.; Akberali, P.M.; Kumari, N.S. Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur. J. Med. Chem. 2005, 40, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Kulkarni, G.M.; Vasireddy, N.R.; Dixit, S.S.; Sharma, V.; Chattopadhyaya, J. Design, synthesis and biological evaluation of novel triazole, urea and thiourea derivatives of quinoline against Mycobacterium tuberculosis. Bioorg. Med. Chem. 2009, 17, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.; Gadhav, G.; Shaikh, M.; Kale, R. Clubbed [1,2,3] triazoles by fluorine benzimidazole: A novel approach to H37Rv inhibitors as a potential treatment for tuberculosis. Bioorg. Med. Chem. Lett. 2008, 18, 6244–6247. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Shameem, S.A.; Ganai, B.A. Antimicrobial studies of unsymmetrical bis-1,2,3-triazoles. Org. Med. Chem. Lett. 2012, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jordao, A.K.; Afonso, P.P.; Ferreira, V.F.; de Souza, M.C.; Almeida, C.O.; Beltrame, C.O.; Paiva, D.P.; Wardell, S.M.; Wardell, J.L.; Tiekink, E.R.; et al. Antiviral evaluation of N-amino-1,2,3-triazoles against Cantagalo virus replication in cell culture. Eur. J. Med. Chem. 2009, 44, 3777–3783. [Google Scholar] [CrossRef] [PubMed]
- Attolino, E.; Colombo, L.; Mormino, I.; Allegrini, P. Method for the Preparation of Rufinamide Using 1,3-Dipolar Cycloaddition as the Key Step. EP Patent 230.234 A1, 22 September 2010. [Google Scholar]
- Bonacorso, H.G.; Maraes, M.C.; Luz, F.M.; Quintana, P.S.; Zarrata, N.; Martins, M.A.P. New solventless and metal-free synthesis of the antiepileptic drug 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-carboxamide (Rufinamide) and analogues. Tetrahedron Lett. 2015, 56, 441–444. [Google Scholar] [CrossRef]
- Perisic-Janjic, N.; Kaliszan, R. Reversed-Phase TLC and HPLC Retention Data in Correlation Studies with in Silico Molecular Descriptors and Druglikeness Properties of Newly Synthesized Anticonvulsant Succinimide Derivatives. J. Am. Chem. Soc. 2011, 8, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Jain, R.K.; Maikhuri, J.P.; Shukla, P.K.; Kumar, M.; Roy, A.K.; Patra, A.; Singh, V.; Batra, S. Discovery of substituted isoxazolecarbaldehydes as potent spermicides, acrosin inhibitors and mild anti-fungal agents. Hum. Reprod. 2005, 20, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Juntao, Z.; Wei, T.; Jingjing, Q.; Diya, L.; Yang, L.; Yan, J.; Guoqiang, D.; Qianqian, C.; Youjun, Z.; Ju, Z.; et al. Design and synthesis of phenylisoxazole derivatives as novel human acrosin inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 2802–2806. [Google Scholar]
- Talley, J.J.; Bertenshaw, S.R.; Brown, D.L.; Carter, J.S.; Graneto, M.J.; Kellog, M.S.; Koboldt, C.M.; Yuan, J.; Zhang, Y.Y.; Seibert, K. N-[[(5-Methyl-3-phenylisoxazol-4-yl)-phenyl]sulfonyl]propanamide, Sodium Salt, Parecoxib Sodium: A Potent and Selective Inhibitor of COX-2 for Parenteral Administration. J. Med. Chem. 2000, 43, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, T.; Dileep, J.S.; Walsh, J.C.; Shapiro, A.; Talley, J.J.; Phelps, M.E.; Herschman, H.R.; Barrio, J.R.; Satyamurthy, N. Synthesis of 4-(5-[18F]fluoromethyl-3-phenylisoxazol-4-yl)benzenesulfonamide, a new [18F]fluorinated analogue of valdecoxib, as a potential radiotracer for imaging cyclooxygenase-2 with positron emission tomography. Bioorg. Med. Chem. Lett. 2005, 15, 4699–4702. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.D.; Talley, J.J.; Berterishaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and Biological Evaluation of the 1,5-Diarylpyrazole Class of Cyclooxygenase-2 Inhibitors: Identification of 4-[5-(4-Methylphenyl)-3- (trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Kawakami, T.; Hirano, E.; Yokota, H.; Kitajima, H. Novel Phthalimidine Synthesis. Mannich Condensation of o-Phthalaldehyde with Primary Amines using 1,2,3–1H-Benzotriazole and 2-Mercaptoethanol as Dual Synthetic Auxiliaries. Synlett 1996, 1996, 353–355. [Google Scholar] [CrossRef]
- Anzini, M.; Capelli, A.; Vomero, S.; Giorgi, G.; Langer, T.; Bruni, G.; Romero, M.R.; Basile, A.S. Molecular Basis of Peripheral vs Central Benzodiazepine Receptor Selectivity in a New Class of Peripheral Benzodiazepine Receptor Ligands Related to Alpidem. J. Med. Chem. 1996, 39, 4275–4284. [Google Scholar] [CrossRef] [PubMed]
- Gotor, V.; Limeres, F.; Garcia, R.; Bayod, M.; Brieva, R. Enzymatic resolution of (±)-6-(5-chloropyridin-2-yl)-6-vinyloxy-carbonyloxy-6,7-dihydro[5H]pyrrolo[3,4-b]pyrazin-5-one. Synthesis of (+)-zopiclone. Tetrahedron Asymmetry 1997, 8, 995–997. [Google Scholar] [CrossRef]
- Goa, K.; Heel, R.C. Zopiclone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy as a hypnotic. Drugs 1986, 32, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Alves, D.C.B.; dos Anjos, J.V.; Cavalcante, N.N.M.; Santos, G.K.N.; Navarro, D.A.F.; Srivastava, R.M. Larvicidal isoxazoles: Synthesis and their effective susceptibility towards Aedes aegypti larvae. Bioorg. Med. Chem. 2013, 21, 940–943. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, M.L.; Naffziger, M.R.; Ashburn, B.O.; Zakharov, L.N.; Carter, R.G. Highly regioselective nitrile oxide dipolar cycloadditions with ortho-nitrophenyl alkynes. Org. Biomol. Chem. 2012, 10, 9204–9213. [Google Scholar] [CrossRef] [PubMed]
- Mabrour, M.; Bougrin, K.; Benhida, R.; Loupy, A.; Soufiaoui, M. An efficient one-step regiospecific synthesis of novel isoxazolines and isoxazoles of N-substituted saccharin derivatives through solvent-free microwave-assisted [3+2] cycloaddition. Tetrahedron Lett. 2007, 48, 443–447. [Google Scholar] [CrossRef]
- Askri, M.; Ben Hamadi, N.; Msadek, M.; Rammah, M.E.B. Réactivité des énones cycliques vis-à-vis du 2-diazopropane: Synthèse de spiro-Δ3-(1,3,4)-oxadiazoline et de pyrazoléine. J. Soc. Chim. Tunis. 2006, 8, 219–222. [Google Scholar]
- Boudriga, S.; Askri, M.; Gharbi, R.; Rammah, M.; Ciamala, K. 1.3-Dipolar Cycloadditions of Arylnitrile oxides and Diarylnitrilimines with some 2-Arylidene-1,3-Indanediones. Regiochemistry of the Reactions. J. Chem. Res. 2003, 2003, 204–207. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Rammah, M.M.; Othman, M.; Ciamala, K.; Strohmann, C.; Rammah, M.B. Silver-catalyzed spirolactonization: First synthesis of spiroisoindole-γ-methylene-γ-butyrolactones. Tetrahedron 2008, 64, 3505–3516. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, L.G. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kamalraj, V.R.; Senthil, S.; Kannan, P. One-pot synthesis and the fluorescent behavior of 4-acetyl-5-methyl-1,2,3-triazole regioisomers. J. Mol. Struct. 2008, 892, 210–215. [Google Scholar] [CrossRef]
- Valizadeh, H.; Amiri, M.; Gholipur, H. Efficient and convenient method for the synthesis of isoxazoles in ionic liquid. J. Heterocycl. Chem. 2009, 46, 108–110. [Google Scholar] [CrossRef]
- Coutouli-Argyropoulou, E.; Lianis, P.; Mitakou, P.; Giannoulisa, M.A.; Nowak, J. 1,3-Dipolar cycloaddition approach to isoxazole, isoxazoline and isoxazolidine analogues of C-nucleosides related to pseudouridine. Tetrahedron 2006, 62, 1494–1501. [Google Scholar] [CrossRef]
- Hansen, T.V.; Wu, P.; Fokin, V.V. One-Pot Copper(I)-Catalyzed Synthesis of 3,5-Disubstituted Isoxazoles. J. Org. Chem. 2005, 70, 7761–7764. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B.A. Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef] [PubMed]
- Lo Vecchio, G.; Garssi, G.; Risitano, F.; Foti, F. Unusual reaction of benzonitrile oxide as phenylnitrosocarbene. Tetrahedron Lett. 1973, 14, 3777–3780. [Google Scholar] [CrossRef]
- Crystal data for compound 4a C28H24N2O4 (115 K): Monoclinic, P21/c, a = 14.149(4), b = 17.091(3), c = 23.576(8) Å, β = 126.741(18)°, λ = 0.71073 Å, V = 4569(2) Å3, Z = 8, Dc = 1.316 Mg/m3.
- Creary, X.; Anderson, A.; Brophy, C.; Crowell, F.; Funk, Z. Method for Assigning Structure of 1,2,3-Triazoles. J. Org. Chem. 2012, 277, 8756–8761. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.R.; Lincoln, P.; Norden, B.; Kann, N. Sequential One-Pot Ruthenium-Catalyzed Azide-Alkyne Cycloaddition from Primary Alkyl Halides and Sodium Azide. J. Org. Chem. 2011, 76, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.K.; Boren, B.C.; Fokin, V.V. Ruthenium-Catalyzed Cycloaddition of Aryl Azides and Alkynes. Org. Lett. 2007, 9, 5337–5339. [Google Scholar] [CrossRef] [PubMed]
- Otwinowski, Z.; Minor, W. Methods in Enzymology. In Macromolecular Crystallography, Part A; Carter, C.W., Jr., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- SIR92 Program; Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds not are available from the authors.







| Products a | Conventional Conditions | Ag2CO3-Catalyzed Conditions b | CuI-Catalyzed Conditions b | |||
|---|---|---|---|---|---|---|
| Time (h) | Yield (%) | Time (h) | Yield (%) | Time (h) | Yield (%) | |
| 4a | 48 | 57 | 24 | 73 | 6 | 85 |
| 4b | 120 | 28 | 24 | 58 | 6 | 63 |
| 4c | 48 | 59 | 24 | 70 | 6 | 75 |
| 4d | 72 | 47 | 24 | 50 | 8 | 63 |
| 6a | 48 | 62 | 24 | 73 | 6 | 89 |
| 6b | 72 | 48 | 24 | 58 | 6 | 75 |
| 6c | 48 | 59 | 24 | 70 | 6 | 85 |
| 6d | 48 | 42 | 24 | 53 | 6 | 67 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rammah, M.M.; Gati, W.; Mtiraoui, H.; Rammah, M.E.B.; Ciamala, K.; Knorr, M.; Rousselin, Y.; Kubicki, M.M. Synthesis of Isoxazole and 1,2,3-Triazole Isoindole Derivatives via Silver- and Copper-Catalyzed 1,3-Dipolar Cycloaddition Reaction. Molecules 2016, 21, 307. https://doi.org/10.3390/molecules21030307
Rammah MM, Gati W, Mtiraoui H, Rammah MEB, Ciamala K, Knorr M, Rousselin Y, Kubicki MM. Synthesis of Isoxazole and 1,2,3-Triazole Isoindole Derivatives via Silver- and Copper-Catalyzed 1,3-Dipolar Cycloaddition Reaction. Molecules. 2016; 21(3):307. https://doi.org/10.3390/molecules21030307
Chicago/Turabian StyleRammah, Mohamed Mehdi, Wafa Gati, Hasan Mtiraoui, Mohamed El Baker Rammah, Kabula Ciamala, Michael Knorr, Yoann Rousselin, and Marek M. Kubicki. 2016. "Synthesis of Isoxazole and 1,2,3-Triazole Isoindole Derivatives via Silver- and Copper-Catalyzed 1,3-Dipolar Cycloaddition Reaction" Molecules 21, no. 3: 307. https://doi.org/10.3390/molecules21030307
APA StyleRammah, M. M., Gati, W., Mtiraoui, H., Rammah, M. E. B., Ciamala, K., Knorr, M., Rousselin, Y., & Kubicki, M. M. (2016). Synthesis of Isoxazole and 1,2,3-Triazole Isoindole Derivatives via Silver- and Copper-Catalyzed 1,3-Dipolar Cycloaddition Reaction. Molecules, 21(3), 307. https://doi.org/10.3390/molecules21030307