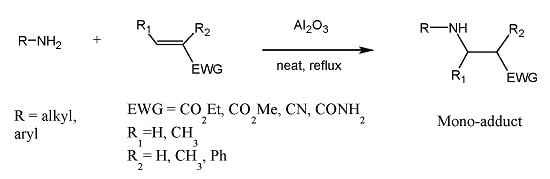
Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Procedure for Preparation of Mono-Adducts
3.3. Product Identification
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perlmutter, P. Conjugate addition reactions in organic synthesis. Tetrahedron 1992, 9, 114–136. [Google Scholar]
- Rulev, A.Y. Aza-Michael reaction: Achievements and prospects. Russ. Chem. Rev. 2011, 80, 197–218. [Google Scholar] [CrossRef]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Cardillo, G.; Tomasini, C. Asymmetric synthesis of beta-amino acids and alpha-substituted beta-amino acids. Chem. Soc. Rev. 1996, 25, 117–128. [Google Scholar] [CrossRef]
- Hradil, P.; Hlavac, J.; Soural, M.; Hajduch, M.; Kolar, M.; Vecerova, R. 3-Hydroxy-2-phenyl-4(1H)-quinolinones as promising biologically active compounds. Mini Rev. Med. Chem. 2009, 9, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Ramesh Reddy, A.; Gopal Rao, Y.; Narsaiah, A.V.; Reddy, B.V.S. Lanthanum trichloride (LaCl3): An efficient catalyst for conjugate addition of amines to electron-deficient olefins. Lett. Org. Chem. 2007, 4, 462–464. [Google Scholar]
- Duan, Z.; Xuan, X.; Li, T.; Yang, C.; Wu, Y. Cerium (IV) ammonium nitrate (CAN) catalyzed aza-Michael addition of amines to α,β-unsaturated electrophiles. Tetrahedron Lett. 2006, 47, 5433–5436. [Google Scholar] [CrossRef]
- Meshram, H.M.; Lakshindra, C.; Reddy, P.N.; Sadashiv, K.; Yadav, J.S. Zirconium(IV) chloride-mediated chemoselective conjugate addition of aliphatic amines to α,β-ethylenic compounds. Synth. Commun. 2006, 36, 795–801. [Google Scholar] [CrossRef]
- Yadav, J.S.; Ramesh Reddy, A.; Gopal Rao, Y.; Narsaiah, A.V.; Subba Reddy, B.V. Samarium(III) triflate catalyzed conjugate addition of amines to electron-deficient alkenes. Synthesis 2007, 3447–3450. [Google Scholar] [CrossRef]
- Vijender, M.; Kishore, P.; Satyanarayana, B. Cadmium chloride (CdCl2): An efficient catalyst for conjugate addition of amines to electron-poor alkenes. Synth. Commun. 2007, 37, 591–594. [Google Scholar] [CrossRef]
- Ying, A.; Wang, L.; Deng, H.; Chen, J.; Chen, X.; Ye, W. Aza-Michael addition of aliphatic or aromatic amines to α,β-unsaturated compounds catalyzed by a DBU-derived ionic liquid under solvent-free conditions. Tetrahedron Lett. 2009, 50, 1653–1657. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Y.; Guo, S.-R. Silica sulfuric acid promotes aza-Michael addition reactions under solvent-free conditions as a heterogeneous and reusable catalyst. Molecules 2009, 14, 4779–4789. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Bonifácio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalystfree inwater, onwater, green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Bonifácio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Solventfree and catalystfree chemistry: A benign pathway to sustainability. ChemSusChem 2014, 7, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Likhar, P.R.; Kantam, M.L.; Bhargava, S. Polyaniline-supported metal catalysts for green synthesis. Indian J. Chem. Sect. A 2012, 51A, 155–165. [Google Scholar]
- Dai, L.; Zhang, Y.; Dou, Q.; Wang, X.; Chen, Y. Chemo/regioselective aza-Michael additions of amines to conjugate alkenes catalyzed by polystyrene-supported AlCl3. Tetrahedron 2013, 69, 1712–1716. [Google Scholar] [CrossRef]
- Nguyen, L.T.L.; Nguyen, T.T.; Nguyen, K.D.; Phan, N.T.S. Metal-organic framework MOF-199 as an efficient heterogeneous catalyst for aza-Michael reaction. Appl. Catal. A Gen. 2012, 425–426, 44–52. [Google Scholar] [CrossRef]
- Verma, S.; Mungse, H.P.; Kumar, N.; Choudhary, S.; Jain, S.L.; Sain, B.; Khatri, O.P. Graphene oxide: An efficient and reusable carbocatalyst for aza-Michael addition of amines to activated alkenes. Chem. Commun. 2011, 47, 12673–12675. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.P.; Silva, M.E.; Rodrigues, L.M.; Oliveira-Campos, A.M.F.; Hrdina, R. Aza-Michael reactions with vinyl sulfones and Amberlyst-15 as catalyst. Tetrahedron Lett. 2007, 48, 9040–9043. [Google Scholar] [CrossRef]
- Ai, X.; Wang, X.; Liu, J.; Ge, Z.; Cheng, T.; Li, R. An effective aza-Michael addition of aromatic amines to electron-deficient alkenes in alkaline Al2O3. Tetrahedron 2010, 66, 5373–5377. [Google Scholar] [CrossRef]
- Xu, H.; Liao, W.M.; Li, H.F. A mild and efficient ultrasound-assisted synthesis of diaryl ethers without any catalyst. Ultrason. Sonochem. 2007, 14, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Bosica, G.; Gabarretta, J. Unprecedented one-pot multicomponent synthesis of propargylamines using Amberlyst A-21 supported CuI under solvent-free conditions. RSC Adv. 2015, 5, 46074–46087. [Google Scholar] [CrossRef]
- Bosica, G.; Spiteri, J.; Borg, C. Aza-Michael reaction: Selective mono- versus bis-addition under environmentally friendly conditions. Tetrahedron Lett. 2014, 70, 2449–2454. [Google Scholar] [CrossRef]
- Bosica, G.; Debono, A.J. Uncatalyzed, green aza-Michael addition of amines to dimethyl maleate. Tetrahedron 2014, 70, 6607–6612. [Google Scholar] [CrossRef]
- Hoganson, E.D.E. Photocycloaddition Reactions of trans-β-nitrostyrene. Ph.D. Thesis, Iowa State University of Science and Technology, Ames, IA, USA, 1965. [Google Scholar]
- Ellman, J.A.; Bergman, R.G.; Colby, D.A. Stereoselective alkylation of α,β-unsaturated imines via C-H bond activation. J. Am. Chem. Soc. 2006, 128, 5604–5605. [Google Scholar]
- Moringa, H.; Morikawa, H.; Sudo, A.; Endo, T. A new water-soluble branched poly(ethylene imine) derivative having hydrolyzable imidazolidine moieties and its application to long-lasting release of aldehyde. J. Polym. Sci. A1 2010, 48, 4529–4536. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Liu, L.; Zu, H.; Wang, Y. RuCl3 in poly(ethylene glycol): A highly efficient and recyclable catalyst for the conjugate addition of nitrogen and sulfur nucleophiles. Synthesis 2005, 13, 2129–2136. [Google Scholar] [CrossRef]
- Wendelin, W.; Riedl, R. N1-and N2-Substituted 2-Amino-5,6-dihydro-4(1H)-pyrimidinones. Monatsh. Chem. 1985, 116, 237–252. [Google Scholar] [CrossRef]
- Player, M.R.; Huang, H.; Hutta, D.A. 5-oxo-5,8-dihydro-pyridopyrimidines as Inhibitors of C-FMS Kinase. US Patent 2007/60577 A1, 2007. [Google Scholar]
- Sawant, D.P.; Justus, J.; Balasubramanian, V.V.; Ariga, K.; Srinivasu, P.; Velmathi, S.; Halligudi, S.B.; Vinu, A. Heteropoly acid encapsulated SBA-15/TiO2 nanocomposites and their unusual performance in acid-catalysed organic transformations. Chem. Eur. J. 2008, 14, 3200–3212. [Google Scholar] [CrossRef] [PubMed]
- Steuneberg, P.; Sijm, M.; Zuilhof, H.; Sanders, J.P.M.; Scott, E.L.; Frannsen, M.C.R. Lipase-catalyzed aza-Michael reaction on acrylate derivatives. J. Org. Chem. 2013, 78, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.D.; Connell, M.A.; Cudahy, M.M.; Evans, B.R.; May, P.D.; Meglasson, M.D.; Sullivan, T.J.; Schostarez, H.J.; Sih, J.C.; Stevens, F.C.; et al. Synthesis and biological activity of analogues of the antidiabetic/antiobesity agent 3-guanidinopropionic acid: discovery of a novel aminoguanidinoacetic acid antidiabetic agent. J. Med. Chem. 2001, 44, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Elan Pharmaceuticals, Inc.; Pharmacia and Up John Company. 2-amino and 2-thio-substituted 1,3-diaminopropanes. U.S. Patent WO2005/95326 A2, 12 October 2005. [Google Scholar]
- Banno, Y.; Miyamoto, Y.; Sasaki, M.; Oi, S.; Asakawa, T.; Kataoka, O.; Takeuchi, K.; Suzuki, N.; Ikedo, K.; Kosaka, T.; et al. Identification of 3-aminomethyl-1,2-dihydro-4-phenyl-1-isoquinolones: A new class of potent, selective, and orally active non-peptide dipeptidyl peptidase IV inhibitors that form a unique interaction with Lys554. Bioorg. Med. Chem. 2011, 19, 4953–4970. [Google Scholar] [CrossRef] [PubMed]
- Neogi, S.; Naskar, D. One-pot reductive mono-n-alkylation of aromatic nitro compounds using nitriles as alkylating reagents. Synthetic Commun. 2011, 41, 1901–1915. [Google Scholar] [CrossRef]
- Douraki, S.M.; Massah, A.R. The Zeolite ZSM-5-SO3H catalyzed aza-Michael addition of amines and sulphonamides to electron-deficient alkenes under solvent-free conditions. Indian J. Chem. 2015, 54B, 1346–1349. [Google Scholar]
- Surrey, A.R. 4-Thiazolidones. IV. The Preparation of Some 3-Alkylaminoalkyl-2-aryl Derivatives. J. Am. Chem. Soc. 1949, 71, 3354–3356. [Google Scholar] [CrossRef]
- Zhou, Y.; Porco, J.A.; Snyder, J.K. Synthesis of 5,6,7,8-tetrahydro-1,6-naphthyridines and related heterocycles by cobalt-catalyzed [2 + 2 + 2] cyclizations. Org. Lett. 2007, 9, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Biggs, C.; Selley, T. Potential hypotensive compounds: Substituted 3-aminopropionates and 3-aminopropionohydroxamic acids. J. Pharm. Sci. 1972, 61, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Halimehjani, A.Z.; Saeedeh, T.; Vahid, A.; Behrouz, N.; Mohammed, R.S. Synthesis and characterization of metal dithiocarbamate derivatives of 3-((pyridin-2-yl)methylamino)propanenitrile: Crystal structure of [3-((pyridin-2-yl)methylamino)propanenitrile dithiocarbamato] nickel(II). Polyhedron 2015, 102, 643–648. [Google Scholar] [CrossRef]
- Link, N.P.; Diaz, J.E.; Orelli, L.R. An efficient synthesis of N-arylputrescines and cadaverines. Synlett 2009, 5, 751–754. [Google Scholar]
- Hoetling, S.; Halberlag, B.; Tamm, M.; Collatz, J.; Mack, P.; Steidle, J.L.M.; Vences, M.; Schulz, S. Identification and synthesis of macrolide pheromones of the grain beetle Oryzaephilus surinamensis and the frog Spinomantis aglavei. Chem. Eur. J. 2014, 20, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- Hebbache, H.; Jerphagnon, T.; Hank, Z.; Bruneau, C.; Renaud, J. Hydrogenation of β-N-substituted enaminoesters in the presence of ruthenium catalysts. J. Organomet. Chem. 2010, 695, 870–874. [Google Scholar] [CrossRef]
- Zamora, R.; Delgado, R.M.; Hidalgo, F.J. Model reactions of acrylamide with selected amino compounds. J. Agr. Food Chem. 2010, 58, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

| Entry | Amine (1) | Yield a 3:4 (%) | Temperature (°C) | Time (h) b |
|---|---|---|---|---|
| 1 | n-C4H9NH2 (1a) | 78:3 | 75 | 3 |
| 2 | n-C6H13NH2 (1b) | 76:4 | 70 | 3 |
| 3 | NH2CH(CH3)CH2CH2OH (1c) | 66:0 | 85 | 3.5 |
| 4 | c-C5H9NH2 (1d) | 90:1 | 75 | 3 |
| 5 | CH2=CHCH2NH2 (1e) | 70:8 | 45 | 3 |
| 6 | CHCCH2NH2 (1f) | 93:0 | 75 | 3 |
| 7 |  (1g) (1g) | 10:0 | 90 | 68 |
| 8 | PhNH2 (1h) | 93:0 | 115 | 5 |
| 9 | BnNH2 (1i) | 80:10 | 95 | 3 |
| 10 | p-C2H5-C6H4NH2 (1j) | 89:0 | 90 | 4 |
| 11 | p-CH3O-C6H4NH2 (1k) | 98:0 | 95 | 3 |
| 12 | NH2CH(CH3)CH2CH3 (1l) | 95:2 | 70 | 4 |

| Entry | Amine (1) | Yield 6:7 (%) a | Temperature (°C) | Time (Hours) b |
|---|---|---|---|---|
| 1 | CH2=CHCH2NH2 (1e) | 76 : 5 | 45 | 3 |
| 2 | CHCCH2NH2 (1f) | 84 : 8 | 75 | 3.5 |
| 3 | p-CH3O -C6H4 CH2NH2 (1m) | 91 : 0 | 90 | 4 |

| Entry | Acceptor | Amine (1) | Yield a (%) (Mono-Adduct) | Temperature (°C)/Time (h) b |
|---|---|---|---|---|
| 1 | Acrylonitrile (8) | n-C6H13NH2 (1b) | 98 (9b) | 70/4 |
| 2 | 8 | c-C5H9NH2 (1d) | 92 (9d) | 65/3.5 |
| 3 | 8 | CHCCH2NH2 (1f) | 83 (9f) | 75/3.5 |
| 4 | 8 | PhNH2 (1h) | 90 (9h) | 95/3 |
| 5 | 8 |  (1n) (1n) | 92 (9n) | 70/4 |
| 6 | 8 | p-CH3OPhCH2NH2 (1o) | 100 (9o) | 90/4 |
| 7 | 8 | CH(CH3)2NH2 (1p) | 95 (9p) | −20/3 |
| 8 | Acrylamide (10) | n-C4H9NH2 (1a) | 95 (11a) | 75/6 |
| 9 | 10 | PhNH2 (1h) | 90 (11h) | 95/7 |
| 10 | Methyl methacrylate (12) | n-C4H9NH2 (1a) | 68 (13a) | 75/5 |
| 11 | 12 | n-C6H13NH2 (1b) | 78 (13b) | 90/5 |
| 12 | 12 | n-C5H11NH2 (1q) | 76 (13q) | 90/5 |
| 13 | Methyl trans-crotonate (14) | n-C4H9NH2 (1a) | 71 (15a) | 75/6 |
| 14 | Methyl trans-cinnamate (16) | n-C4H9NH2 (1a) | 43 (17a) | 70/48 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosica, G.; Abdilla, R. Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions. Molecules 2016, 21, 815. https://doi.org/10.3390/molecules21060815
Bosica G, Abdilla R. Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions. Molecules. 2016; 21(6):815. https://doi.org/10.3390/molecules21060815
Chicago/Turabian StyleBosica, Giovanna, and Roderick Abdilla. 2016. "Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions" Molecules 21, no. 6: 815. https://doi.org/10.3390/molecules21060815
APA StyleBosica, G., & Abdilla, R. (2016). Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions. Molecules, 21(6), 815. https://doi.org/10.3390/molecules21060815