Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes
Abstract
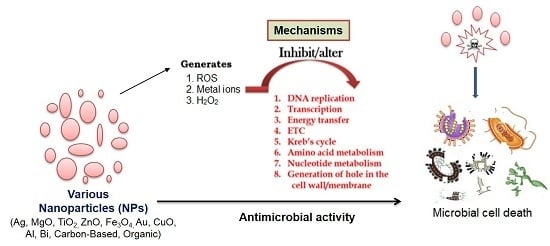
:1. Introduction
2. Nanoparticles/Nanocomposites
2.1. Inorganic NPs with Antibacterial and Antifungal Activities
2.1.1. Silver NPs (AgNPs)
2.1.2. Magnesium Oxide (MgO) NPs
2.1.3. Titanium Dioxide (TiO2) NPs
2.1.4. Zinc Oxide (ZnO) NPs
2.1.5. Iron Oxide (Fe3O4) NPs
2.1.6. Gold (Au) NPs
2.1.7. Copper Oxide (CuO) NPs
2.1.8. Aluminum (Al) NPs
2.1.9. Bismuth (Bi) NPs
2.1.10. Carbon-Based NPs
2.2. Organic NPs
2.2.1. Quaternary Ammonium Compounds
2.2.2. Triclosan and Polysiloxanes
2.2.3. Chitosan
2.3. Antiviral Properties of NPs
3. Biological Compatibility of Nanoparticles (NPs)
4. Biodegradability and Encapsulation of Nanoparticles
5. Nanoparticles and Delivery Systems
6. Limitations
7. Conclusions
8. Future Prospects
Acknowledgments
Authors Contribution
Conflicts of Interest
Abbreviations
| Ag2O | Silver oxide |
| AgNPs | Silver nanoparticles |
| Al2O3 | Alumina oxide |
| AuNPs | Gold nanoparticles |
| BINPs | Bismuth nanoparticles |
| BNPs | Biodegradable nanoparticles |
| CaO | Calcium oxide |
| CuO | Copper oxide |
| CuFe2O4 | Copper ferrite |
| HIV-1 | Human immunodeficiency virus 1 |
| HSV-1 | Herpes simplex virus type 1 |
| HRTEM | High-resolution transmission electron microscopy |
| LRTEM | Low-resolution transmission electron microscopy |
| MBC | minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MgO | Magnesium oxide |
| MnFe2O4 | Manganese ferrite |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NPs | Nanoparticles |
| PAC | Poly-alkyl-cyanoacrylates |
| PCL | Poly-ε-caprolactone |
| PEG | Polyethylene glycol |
| PEI | N-alkylated polyethyleneimine |
| PLGA | Poly-d-l-lactide-co-glycolide |
| PLA | Polylactic acid |
| PL | Poly-l-lysine |
| PNA | Peptide analogues of nucleic acids |
| QAC | Quaternary ammonium compounds |
| RNAi | RNA interference |
| ROS | Reactive oxygen species |
| siRNA | Small interfering RNA |
| TiO2 | Titanium dioxide |
| TiO2NPs | Titanium dioxide nanoparticles |
| TEM | Transmission electron microscopy |
| TPGS | Tocopheryl polyethylene glycol 1000 succinate |
| ZnO | Zinc oxide |
| ZnFe2O4 | Zinc ferrite |
References
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Habich, D. Antibacterial natural products in medicinal chemistry—Exodus or revival? Angew. Chem. Int. Ed. 2006, 45, 5072–5129. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug. Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Lemke, T.L.; Williams, D.A. Foye’s Principles of Medicinal Chemistry; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2008. [Google Scholar]
- Verheij, T.J. The antibiotic revolution should be more focused. Br. J. Gen. Pract. 2009, 59, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Sneddon, J.; Nathwani, D. Overview of strategies for overcoming the challenge of antimicrobial resistance. Expert. Rev. Clin. Pharmacol. 2010, 3, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Kamal, Y.E.S.; Aziz, R.; Sarmidi, M.R.; El-Enshasy, H.A. Antibiotics as microbial secondary metabolites: Production and application. J. Teknol. 2012, 59, 101–111. [Google Scholar]
- Boman, H.G. Innate immunity and the normal micro flora. Immunol. Rev. 2000, 173, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Huttner, K.M. Antimicrobial Peptides: An Emerging Concept in Cutaneous Biology. J. Investig. Dermatol. 1998, 111, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial Peptides: Versatile Biological Properties. Int. J. Pept. 2013. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Malik, M.; Kernsm, R.J.; Zhaom, X. Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 2008, 52, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Eko, K.E.; Forshey, B.M.; Carrel, M.; Schweizer, M.L.; Perencevich, E.N.; Smith, T.C. Molecular characterization of methicillin-resistant Staphylococcus aureus (MRSA) nasal colonization and infection isolates in a Veterans Affairs hospital. Antimicrob. Resist. Infect. Control. 2015. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.; Edwards, R. Insights into antibiotic resistance through metagenomic approaches. Future Microbiol. 2012, 7, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Crumplin, G.C.; Odell, M. Development of resistance to ofloxacin. Drugs 1987, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L.; Baquero, F. Mutation frequencies and antibiotic resistance. Antimicrob. Agents Chemother. 2000, 44, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Hegstad, K.; Mikalsen, T.; Coque, T.M.; Werner, G.; Sundsfjord, A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 2010, 16, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.P.; Balumahendiran, M.; Suryanarayana, V.V.S.; Raghavendra, A.G.; Shome, B.R.; Ganjendragad, M.R.; Prabhudas, K. PCR-based diagnosis of surra-targeting VSG gene: Experimental studies in small laboratory rodents and buffalo. Vet. Parasitol. 2010, 171, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, G.R.; Sengupta, P.P.; Balamurugan, V.; Prabhudas, K.; Rahman, H. PCR based diagnosis of trypanosomiasis exploring invariant surface glycoprotein (ISG) 75 gene. Vet. Parasitol. 2013, 193, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, B.W.; Wang, C.; Johnson, C.M.; Kaltenboeck, B.; Boothe, D.M. Detection of fluoroquinolone resistance level in clinical canine and feline Escherichia coli pathogens using rapid real-time PCR assay. Vet. Microbiol. 2009, 139, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Volokhov, D.; Chizhikov, V.; Chumakov, K.; Rasooly, A. Microarray analysis of erythromycin resistance determinants. J. Appl. Microbiol. 2003, 95, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.X.; Zhang, Z.W.; Wang, C.; Yang, H.W.; Jiang, D.; Zhang, Q.; Mitchelson, K.; Cheng, J. Use of a DNA microarray for simultaneous detection of antibiotic resistance genes among staphylococcal clinical isolates. J. Clin. Microbiol. 2007, 45, 3514–3521. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Moe, L.A.; Rodbumrer, J.; Gaarder, A.; Handelsman, J. Functional metagenomics reveals diverse β-lactamases in a remote Alaskan soil. ISME J. 2009, 3, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Donato, J.J.; Moe, L.A.; Converse, B.J.; Smart, K.D.; Berklein, F.C.; McManus, P.S. Metagenomic analysis of apple orchard soil reveals antibiotic resistance genes encoding predicted bifunctional proteins. Appl. Environ. Microbiol. 2010, 76, 4396–4401. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Mizuta, S.; Suenaga, H.; Miyazaki, K. Metagenomic screening for bleomycin resistance genes. Appl. Environ. Microbiol. 2008, 74, 6803–6805. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; Greko, C. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Akhtar, M.; Swamy, M.K.; Umar, A.; Sahli, A.; Abdullah, A. Biosynthesis and characterization of silver nanoparticles from methanol leaf extract of Cassia didymobotyra and assessment of their antioxidant and antibacterial activities. J. Nanosci. Nanotechnol. 2015, 15, 9818–9823. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.Y.; Bhatia, S.N.; Toner, M. Nanotechnology: Emerging tools for biology and medicine. Genes Dev. 2013, 27, 2397–2408. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.; Mishra, S.; Jena, S.; Mishra, S.S. Nanotechnology-future prospect in recent medicine: A review. Int. J. Basic Clin. Pharmacol. 2013, 2, 353–359. [Google Scholar] [CrossRef]
- Fröhlich, E.; Salar-Behzadi, S. Toxicological assessment of inhaled nanoparticles: Role of in vivo, ex vivo, in vitro, and in silico studies. Int. J. Mol. Sci. 2014, 15, 4795–4822. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar Rai, V.; Jamuna Bai, A. Nanoparticles and their potential application as antimicrobials. In Science against Microbial Pathogen: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 2, pp. 197–209. [Google Scholar]
- Hajipour, J.M.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trend Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R. There’s plenty of room at the bottom. Science 1991, 254, 1300–1301. [Google Scholar]
- Adibkia, K.; Omidi, Y.; Siahi, M.R.; Javadzadeh, A.R.; Barzegar-Jalali, M.; Barar, J.; Maleki, N.; Mohammadi, G.; Nokhodchi, A. Inhibition of endotoxin-induced uveitis by methylprednisolone acetate nanosuspension in rabbits. J. Ocul. Pharmacol. Ther. 2007, 23, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Zinjarde, S.S. Bio-inspired nanomaterials and their applications as antimicrobial agents. Chronic Young Sci. 2012, 3, 74–81. [Google Scholar] [CrossRef]
- Bahrami, K.; Nazari, P.; Nabavi, M.; Golkar, M.; Almasirad, A.; Shahverdi, A.R. Hydroxyl capped silver-gold alloy nanoparticles: Characterization and their combination effect with different antibiotics against Staphylococcus aureus. Nanomed. J. 2014, 1, 155–161. [Google Scholar]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar]
- Nam, J.M.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Osterfeld, S.J.; Yub, H.; Gaster, R.S.; Caramuta, S.; Xu, L.; Han, S.J.; Hall, D.A.; Wilson, R.J.; Sun, S.; White, R.L.; et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA 2008, 105, 20637–20640. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtarc, M.S.; Mohantya, S.K.; Sinniah, U.R. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spec. Acta Part A Mol. Biomol. Spectr. 2015, 151, 939–944. [Google Scholar]
- Haider, A.; Kang, I.K. Preparation of silver nanoparticles and their industrial and biomedical applications: A comprehensive review. Adv. Mater. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green chemistry based benign routes for nanoparticle synthesis. J. Nanopar. 2014. [Google Scholar] [CrossRef]
- Tanja, K.; Ralph, J.; Eva, O.; Claes-Göran, G. Silver-based crystalline nanoparticles, microbially fabricated. PNAS 1999, 96, 13611–13614. [Google Scholar]
- Fu, J.K.; Zhang, W.D.; Liu, Y.Y.; Lin, Z.Y.; Yao, B.X.; Weng, S.Z.; Zeng, J.L. Characterization of adsorption and reduction of noble metal ions by bacteria. Chem. J. Chin. Univ. 1999, 20, 1452–1454. [Google Scholar]
- El-Shanshoury, A.E.R.; ElSilk, S.E.; Ebeid, M.E. Extracellular biosynthesis of silver nanoparticles using Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, and Streptococcus thermophilus ESh1 and their antimicrobial activities. ISRN Nanotechnol. 2011. [Google Scholar] [CrossRef]
- Bhainsa, C.K.; D’Souza, F.S. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus funigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.; Ingle, A.; Bawaskar, M.; Rai, M. Fusarium solani: A novel biological agent for extracellular synthesis of nanoparticles. J. Nanopart. Res. 2009, 11, 2079–2085. [Google Scholar]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, K.; Dillip, G.R.; Narasimha, G.; John Sushma, N.; Deva Prasad Raju, B. Phytofabrication and characterization of silver nanoparticles from Piper betle broth. Res. J. Nanosci. Nanotechnol. 2012, 2, 17–23. [Google Scholar]
- Swamy, M.K.; Sudipta, K.M.; Jayanta, K.; Balasubramanya, S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl. Nanosci. 2015, 5, 73–81. [Google Scholar] [CrossRef]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2. [Google Scholar] [CrossRef]
- Mashwani, Z.R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Jana, A.D.; Samanta, S.K.; Mostafa, G. Facile synthesis of silver nanoparticles with highly efficient anti-microbial property. Polyhedron 2007, 26, 4419–4426. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G. Ramachandra Rao P, Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of Gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Tapia, J.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Manivannan, G.; Kim, S.J.; Jeyasubramanian, K.; Premanathan, M. Antibacterial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. J. Nano Res. 2012, 14. [Google Scholar] [CrossRef]
- Koper, O.; Klabunde, J.; Marchin, G.; Klabunde, K.J.; Stoimenov, P.; Bohra, L. Nanoscale powders and formulations with biocidal activity toward spores and vegetative cells of Bacillus species, viruses, and toxins. Curr. Microbiol. 2002, 44, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, K.; Mori, M.; Yoshida, M.; Oikawa, S.; Kawanishi, S. Photo-irradiated titanium dioxide catalyzes site specific DNA damage via generation of hydrogen peroxide. Free Radic. Res. 2004, 38, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; de Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2011, 3, 643–646. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.A.; El-Tras, W.F.; Moussa, S.; El-Baz, A.F.; Mahrous, H.; Salem, M.F.; Brimer, L. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens. J. Food Saf. 2011, 31, 211–218. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, ZQ.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Gil-Tomas, J. Lethal photosensitisation of Staphylococcus aureus using a toluidine blue O-tiopronin-gold nanoparticles conjugate. J. Mater. Chem. 2007, 17, 3739–3746. [Google Scholar] [CrossRef]
- Pissuwan, D.; Cortie, C.H.; Valenzuela, S.M.; Cortie, M.B. Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol. 2009, 28, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Perni, S. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials 2009, 30, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef]
- Sampath, M.; Vijayan, R.; Tamilarasu, E.; Tamilselvan, A.; Sengottuvelan, B. Green synthesis of novel jasmine bud-shaped copper nanoparticles. J. Nanotechnol. 2014, 2014. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Sarkar, R.K.; Chattopadhyay, A.P.; Aich, P.; Chakraborty, R.; Basu, T. A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology 2012, 23, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; ulHasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Rupareli, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–771. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Patra1, J.K.; Pramanik, K.; Panda, N.; Thatoi, H. Characterization and Evaluation of antibacterial activities of chemically synthesized Iron Oxide nanoparticles. World J. Nano Sci. Eng. 2012, 2, 196–200. [Google Scholar] [CrossRef]
- Sadiq, M.; Chowdhury, B.; Chandrasekaran, N.; Mukherjee, A. Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hossain, M.; Wang, C.; Qiao, Y.; An, J.; Ma, L.; Su, M. Targeted nanoparticles for enhanced X-ray radiation killing of multi drug resistant bacteria. Nanoscale 2013, 5, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Nazari, P.; Dowlatabadi-Bazaz, R.; Mofid, M.R.; Pourmand, M.R.; Daryani, N.E.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shahverdi, A.R. The Antimicrobial effects and metabolomic footprinting of carboxyl-capped bismuth nanoparticles against Helicobacter pylori. Appl. Biochem. Biotechnol. 2013, 172, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter. Langmuir 2008, 24, 6409–6013. [Google Scholar]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial activity of single-walled carbon nanotubes: Length effect. Langmuir 2010, 26, 6013–6019. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmacol. 2012, 261, 121–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Henderson, A.; Field, C. Antimicrobial activity of single-walled carbon nanotubes suspended in different surfactants. J. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Leid, J.; Ditto, A.; Knapp, A.; Shah, P.; Wright, B.; Blust, R.; Christensen, L.; Clemons, C.B.; Wilber, J.P.; Young, G.W.; et al. In vitro antimicrobial studies of silver carbine complexes: Activity of free and nanoparticle carbene formulations against clinical isolates of pathogenic bacteria. J. Antimicrob. Chemother. 2012, 67, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Núnez, N.V.; Turrent, L.D.C.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Boegli, L.; James, G.; Velasquillo, C.; Sánchez-Sánchez, R.; Martínez-Martínez, R.E.; Martínez-Castañón, G.A.; Martinez-Gutierrez, F. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallmann, E.J.J.; Cunha, F.A.; Castro, B.N.; Maciel, A.M.; Menezes, E.A.; Fechine, P.B.A. Antifungal activity of silver nanoparticles obtained by green synthesis. Rev. Inst. Med. Trop. Sao. Paul. 2015, 57, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci. Total Environ. 2015, 521, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Sundrarajan, M.; Suresh, J.; Gandhi, R.R. A comparative study on antibacterial properties of MgO nanoparticles prepared under different calcinations temperature. Dig. J. Nanomater. Biostruct. 2012, 7, 983–989. [Google Scholar]
- Fang, M.; Chen, J.H.; Xu, X.L.; Yang, P.H.; Hildebrand, H.F. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int. J. Antimicrob. Agents 2006, 27, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Koodali, R.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticles suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Manna, A.C. Synthesis, Characterization, and Antimicrobial Activity of Zinc Oxide Nanoparticles. In Nano-Antimicrobials: Progress and Prospects; Cioffi, N., Rai, M., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; pp. 151–180. [Google Scholar]
- Tang, Z.X.; Feng, B. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J. On the synthesis and optical absorption studies of nano-size magnesium oxide powder. J. Phys. Chem. Sol. 2008, 69, 2764–2772. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Kumar, R.T.; Kennedy, L.J.; Vijaya, J.J. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nanostructures. J. Alloy Comp. 2011, 509, 9809–9815. [Google Scholar] [CrossRef]
- Jin, T.; He, Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanopart. Res. 2011, 13, 6877–6885. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.Q.; Lin, Y.J.; Wei, M.; Evans, D.G.; Duan, X. Controllable preparation of Nano-MgO and investigation of its bactericidal properties. J. Inorg. Biochem. 2005, 99, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.; Li, W.; Decker, S.; Davidson, C.; Koper, O.; Zaikovski, V.; Volodin, A.; Rieker, T.; Klabunde, K. Consolidation of metal oxide nanocrystals. Reactive pellets with controllable pore structure that represent a new family of porous, inorganic materials. J. Am. Chem. Soc. 2000, 122, 4921–4925. [Google Scholar] [CrossRef]
- Zeng, H.C. Preparation and integration of nanostructured titanium dioxide. Curr. Opin. Chem. Eng. 2011, 1, 11–17. [Google Scholar] [CrossRef]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiest, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO(2) and Ag(2)O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 8, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Liu, Z.P.; Liu, L.; Gao, W. Mechanism and activity of photocatalytic oxygen evolution on titania anatase in aqueous surroundings. J. Am. Chem. Soc. 2010, 132, 13008–13015. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Chu, W.C.; Sun, D.S.; Huang, H.S.; Chen, J.H.; Tsai, P.J.; Lin, N.T.; Yu, M.S.; Hsu, S.F.; Wang, S.L.; Chang, H.H. Visible-light-induced bactericidal activity of a nitrogen-doped titanium photocatalyst against human pathogens. Appl. Environ. Microbiol. 2006, 72, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Xie, R.; Imlay, K.; Shang, J.K. Visible-light-induced bactericidal activity of titanium dioxide codoped with nitrogen and silver. Environ. Sci. Technol. 2010, 44, 6992–6997. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.P.; Huang, Y.T.; Yang, S.C. The antifungal efficacy of nano-metals supported TiO2 and ozone on the resistant Aspergillus niger spore. J. Hazard. Mater. 2013, 261, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Vidic, J.; Stankic, S.; Haque, F.; Ciric, D.; Le Goffic, R.; Vidy, A.; Jupille, J.; Delmas, B. Selective antibacterial effects of mixed ZnMgO nanoparticles. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Sasidharan, A.; Rani, V.D.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009, 20, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Mehta, R.K.; Mohanty, S.; Padhi, A.; Sengupta, M.; Vaseeharan, B.; Goswami, C.; Sonawane, A. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomed. Nanotech. Biol. Med. 2014, 10, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Chen, W.J.; Tsai, P.J.; Chen, Y.C. Functional Fe3O4/TiO2 core/shell magnetic nanoparticles as photokilling agents for pathogenic bacteria. Small 2008, 4, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Mir, A.; Mallik, D.; Sinha, A.; Nayar, S.; Webster, T.J. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int. J. Nanomed. 2010, 5, 277–283. [Google Scholar]
- Prabhu, Y.T.; Rao, K.V.; Kumari, B.S.; Kumar, V.S.S.; Pavani, T. Synthesis of Fe3O4 nanoparticles and its antibacterial application. Int. Nano Lett. 2015, 5, 85–92. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef]
- Taylor, E.N.; Webster, T.J. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int. J. Nanomed. 2009, 4, 145–152. [Google Scholar]
- Arokiyaraj, S.; Saravanan, M.; Prakash, N.U.; Arasu, M.V.; Vijayakumar, B.; Vincent, S. Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: An in vitro study. Mater. Res. Bull. 2013, 8, 3323–3327. [Google Scholar] [CrossRef]
- Anghel, I.; Grumezescu, A.M.; Andronescu, E.; Anghel, A.G.; Ficai, A.; Saviuc, C.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. Magnetite nanoparticles for functionalized textile dressing to prevent fungal biofilms development. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, C.; Grumezescu, V.; Grumezescu, A.M.; Saviuc, C.; Lazăr, V.; Andronescu, E. Hybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, Supramolecular Chemistry, Quantum-size Related Properties, and Applications towards Biology, Catalysis and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles—Properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Mukhopadhyay, D.; Mukherjee, P. Fabrication of Gold Nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2009, 62, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.H.; Azzay, H.M. Gold nanoparticles for molecular diagnosis. Expert Rev. Mol. Diagn. 2009, 9, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Rasch, M.R.; Konstantin, V.; Brian, A. Limitations on the optical tunability of small diameter Gold nanoshells. Langmuir 2009, 25, 11777–11785. [Google Scholar] [CrossRef] [PubMed]
- Alex, S.; Tiwari, A. Functionalized gold nanoparticles: Synthesis, properties and applications—A review. J.Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of Gold nanorods (NRs) using seed-nediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Badri Narayanan, K.; Sakthivel, N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater. Lett. 2008, 62, 4588–4590. [Google Scholar] [CrossRef]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf. B 2009, 68, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Zubair, S.; Tufail, S.; Sherwani, A.; Sajid, M.; Raman, S.C.; Azam, A.; Owais, M. Fungus-mediated biological synthesis of gold nanoparticles: Potential in detection of liver cancer. Int. J. Nanomed. 2011, 6, 2305–2319. [Google Scholar]
- Bapista, P.; Periera, E.; Eaton, P.; Doria, G.; Miranda, A.; Gomes, I.; Quaresma, P.; Franco, R. Gold nanoparticles for the development of clinical diagnosis methods. Ann. Bioannal. Chem. 2008, 391, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Zharov, V.P. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ho, P.L.; Tong, E.; Wang, L.; Xu, B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003, 3, 1261–1263. [Google Scholar] [CrossRef]
- Burygin, G.L. On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Grace, N.A.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—A brief study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Saha, B. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett. 2007, 2, 614–622. [Google Scholar] [CrossRef]
- Khan, S.; Alam, F.; Azam, A.; Khan, A.U. Gold nanoparticles enhance methylene blue-induced photodynamic therapy: A novel therapeutic approach to inhibit Candida albicans biofilm. Int. J. Nanomed. 2012, 7, 3245–3257. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Bresee, J.; Maier, K.E.; Boncella, A.E.; Melander, C.; Feldheim, D.L. Growth inhibition of Staphylococcus aureus by mixed monolayer gold nanoparticles. Small 2011, 7, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Girilal, M.; Mahdy, S.A.; Somsundar, S.S.; Venkatesan, R.; Kalaichelvan, P.T. Vancomycin bound biogenic gold nanoparticles: A different perspective for development of anti VRSA agents. Process Biochem. 2011, 46, 636–641. [Google Scholar] [CrossRef]
- Ahangari, A.; Salouti, M.; Heidari, Z.; Kazemizadeh, A.R.; Safari, A.A. Development of gentamicin-gold nanospheres for antimicrobial drug delivery to Staphylococcal infected foci. Drug Deliv. 2013, 20, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Roshmi, T.; Soumya, K.R.; Jyothis, M.; Radhakrishnan, E.K. Effect of biofabricated gold nanoparticle-based antibiotic conjugates on minimum inhibitory concentration of bacterial isolates of clinical origin. Gold Bull. 2015, 48, 63–71. [Google Scholar] [CrossRef]
- Dasari, T.S.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold (I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Kim, C. Viscosity and thermal conductivity of copper oxide nanofluid dispersed in ethylene glycol. Korea Aust. Rheol. J. 2005, 17, 35–40. [Google Scholar]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W.; Pecharapa, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef]
- Sastry, A.B.S.; Aamanchi, R.K.; Prasad, C.S.R.L.; Murty, B.S. Large-scale green synthesis of Cu nanoparticles. Environ. Chem. Lett. 2013, 11, 183–187. [Google Scholar] [CrossRef]
- Pramanik, A.; Laha, D.; Bhattacharya, D.; Pramanik, P.; Karmakar, P. A novel study of antibacterial activity of copper iodide nanoparticle mediated by DNA and membrane damage. Colloids Surf. B 2012, 96, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Weitz, I.S.; Maoz, M.; Panitz, D.; Eichler, S.; Segal, E. Combination of CuO nanoparticles and fluconazole: Preparation, characterization, and antifungal activity against Candida albicans. J. Nanopart. Res. 2015. [Google Scholar] [CrossRef]
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 2015, 9, 7–12. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Abdulameer, F.A. Synthesis and Antibacterial Activity of CuO Nanoparticles Suspension Induced by Laser Ablation in Liquid. Arab. J. Sci. Eng. 2016, 41, 301–310. [Google Scholar] [CrossRef]
- Martínez Flores, E.; Negrete, J.; Torres Villaseñor, G. Structure and properties of Zn-Al-Cu alloy reinforced with alumina particles. Mater. Des. 2003, 24, 281–286. [Google Scholar] [CrossRef]
- Ghorbani, H.R. A review of methods for synthesis of Al nanoparticles. Orient. J. Chem. 2014, 30, 1941–1949. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mohammed Sadiq, I.; Prathna, T.C.; Chandrasekaran, N. Antimicrobial activity of aluminium oxide nanoparticles for potential clinical applications. Sci. Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 1, 245–251. [Google Scholar]
- Balasubramanyam, A.; Sailaja, N.; Mahboob, M.; Rahman, M.F.; Hussain, S.M.; Grover, P. In vitro mutagenicity assessment ofaluminium oxide nanomaterials using the Salmonella/microsome assay. Toxicol. In Vitro 2010, 24, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Saquib, Q.; Musarrat, J. Interaction of Al2O3 nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Appl. Microbiol. 2014, 116, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Claudio, C.R.; Chellam, S. Bismuth nanoparticles: Antimicrobials of broad-spectrum, low cost and safety. In Nanomedicine; Seifalian, A., de Mel, A., Kalaskar, D.M., Eds.; One Central Press: Manchester, UK, 2015; pp. 430–437. [Google Scholar]
- Badireddy, A.R.; Hernandez-Delgadillo, R.; Sanchez-Najera, R.I.; Chellam, S.; Cabral-Romero, C. Synthesis and characterization of lipophilic bismuth dimercaptopropanol nanoparticles and their effects on oral microorganisms growth and biofilm formation. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Diaz, D.; Arevalo-Niño, K.; Garza-Enriquez, M.; de la Garza-Ramos, M.A.; Cabral-Romero, C. Zerovalent bismuth nanoparticles inhibit Streptococcus mutans growth and formation of biofilm. Int. J. Nanomed. 2012, 7, 2109–2113. [Google Scholar]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Martinez-Sanmiguel, J.J.; Diaz, D.; Zumeta-Dube, I.; Arevalo-Niño, K.; Cabral-Romero, C. Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. Int. J. Nanomed. 2013, 8, 1645–1652. [Google Scholar]
- Ageitos, J.M.; Chuah, J.A.; Numata, K. Design Considerations for Properties of Nanocarriers on Disposition and Efficiency of Drug and Gene Delivery. In Nanomedicines: Design, Delivery and Detection; Braddock, M., Ed.; Royal Society of Chemistry: London, UK, 2016. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, 17–71. [Google Scholar] [CrossRef]
- Pacurar, M.; Qian, Y.; Fu, W.; Schwegler-Berry, D.; Ding, M.; Castranova, V.; Guo, N.L. Cell permeability, migration, and reactive oxygen species induced by multiwalled carbon nanotubes in human microvascular endothelial cells. J. Toxicol. Environ. Health 2012, 75, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, S. Nanoparticles and Nanodevices in Biological Applications, Lecture Notes in Nanoscale Science and Technology; Springer-Verlag: Berlin/Heidelberg, Germany, 2009; Volume 1, pp. 47–67. [Google Scholar]
- Deryabin, D.G.; Davydova, O.K.; Yankina, Z.Z.; Vasilchenko, A.S.; Miroshnikov, S.A.; Kornev, A.B.; Ivanchikhina, A.V.; Troshin, P.A. The activity of [60] fullerene derivatives bearing amine and carboxylic solubilizing groups against Escherichia coli: A comparative study. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Arias, L.R.; Yang, L. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; da Ros, T. Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 23–50. [Google Scholar]
- Nakamura, S.; Mashino, T. Biological Activities of Water-Soluble Fullerene Derivatives; IOP Publishing: Philadelphia, PA, USA, 2009; Volume 159. [Google Scholar]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Daima, H.K.; Bansal, V. Influence of Physicochemical Properties of Nanomaterials on Their Antibacterial Applications. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Rai, M., Kon, K., Eds.; Academic Press/Elsevier: Boston, MA, USA, 2015. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Qi, X.; Li, P.; Chen, W.N.; Mouad, L.; Chang, M.W.; Leong, S.S.J.; Chan-Park, M.B. High potency and broad-spectrum antimicrobial peptides synthesized via ring-opening polymerization of α-aminoacid-N-carboxyanhydrides. Biomacromolecules 2010, 11, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Yeow, J.T. Antibacterial properties of poly (quaternary ammonium) modified gold and titanium dioxide nanoparticles. J. Nanosci. Nnotechnol. 2012, 12, 4601–4606. [Google Scholar] [CrossRef]
- Song, J.; Kong, H.; Jang, J. Bacterial adhesion inhibition of the quaternary ammonium functionalized silica nanoparticles. Coll. Surf. B 2011, 82, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Yudovin-Farber, I.; Golenser, J.; Beyth, N.; Weiss, E.I.; Domb, A.J. Quaternary ammonium polyethyleneimine: Antibacterial activity. J. Nanomater. 2010, 2010. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Butler, R.; Campbell, N.L.; Long, J.; Tan, B.; Duncalf, D.J.; Foster, A.J.; Hopkinson, A.; Taylor, D.; et al. Formation and enhanced biocidal activity of water-dispersable organic nanoparticles. Nat. Nanotechnol. 2008, 3, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Sauvet, G.; Fortuniak, W.; Kazmierski, K.; Chojnowski, J. Amphiphilic block and statistical siloxane copolymers with antimicrobial activity. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2939–2948. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs. 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Yien, L.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012. [Google Scholar] [CrossRef]
- Khandelwal, N.; Kaur, G.; Kumara, N.; Tiwari, A. Application of silver nanoparticles in viral inhibition: A new hope for antivirals. Dig. J. Nanomater. Biostruct. 2014, 9, 175–186. [Google Scholar]
- Sun, R.W.; Chen, R.; Chung, N.P.; Ho, C.M.; Lin, C.L.; Che, C.M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun. 2005, 40, 5059–5061. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ixtepan-Turrent, L.; Garza-Treviño, E.N.; Rodriguez-Padilla, C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J. Nanobiotechnol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, R.W.; Chen, R.; Hui, C.K.; Ho, C.M.; Luk, J.M.; Lau, G.K.; Che, C.M. Nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [PubMed]
- Mehrbod, P.; Motamed, N.; Tabatabaian, M.; Estyar, R.S.; Amini, E.; Shahidi, M.; Kheiri, M.T. In vitro antiviral effect of nanosilver on Influenza virus. DARU J. Pharm. Sci. 2009, 17, 88–93. [Google Scholar]
- Xiang, D.X.; Chen, Q.; Pang, L.; Zheng, C.L. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Virol. Methods 2011, 78, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of Influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A preliminaryassessment of silver nanoparticles inhibition of Monkeypox virus plaque formation. Nanoscale Res. Lett. 2008, 3, 129–133. [Google Scholar] [CrossRef]
- Sun, L.; Singh, A.K.; Vig, K.; Pillai, S.; Shreekumar, R.; Singh, S.R. Silver nanoparticles inhibit replication of Respiratory Sincitial virus. J. Biomed. Biotechnol. 2008, 4, 149–158. [Google Scholar]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug. Chem. 2009, 20, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small 2010, 6, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Ganesan, S. Gold nanoparticles as an HIV entry inhibitor. Curr. HIV Res. 2012, 10, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Broglie, J.J.; Alston, B.; Yang, C.; Ma, L.; Adcock, A.F.; Chen, W.; Yang, L. Antiviral Activity of Gold/Copper Sulfide Core/Shell Nanoparticles against Human Norovirus Virus-Like Particles. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Manuel, C.S.; Moore, M.D.; Jaykus, L.A. Destruction of the Capsid and Genome of GII.4 Human Norovirus Occurs during Exposure to Metal Alloys Containing Copper. Appl. Environ. Microbiol. 2015, 81, 4940–4946. [Google Scholar] [CrossRef] [PubMed]
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 2012, 113, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Amirkhanov, R.N.; Mazurkova, N.A.; Amirkhanov, N.V.; Zarytova, V.F. Composites of peptide nucleic acids with titanium dioxide nanoparticles. IV. Antiviral activity of nanocomposites containing DNA/PNA duplexes. Russ. J. Bioorg. Chem. 2015, 41, 140–146. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z. Biocompatibility and toxicity of nanoparticles and nanotubes. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Reddy, N.N.; Varaprasad, K.; Ravindra, S. Evaluation of blood compatibility and drug release studies of gelatin based magnetic hydrogel nanocomposites. Colloids Surfaces A 2011, 385, 20–27. [Google Scholar] [CrossRef]
- Li, P.W.; Kuo, T.H.; Chang, J.H.; Yeh, J.M.; Chan, W.H. Induction of cytotoxicity and apoptosis in mouse blastocysts by silver nanoparticles. Toxicol. Lett. 2010, 197, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Loeschner, K.; Mortensen, A. The similar neurotoxic effects of nanoparticulate and ionic silver in vivo and in vitro. Neurotoxicology 2012, 33, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Song, S.W.; Kim, H.Y. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Saf. Health Work 2013, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Van Der Ven, L.T.; Sleijffers, A.; Park, M.V.; Jansen, E.H.; Van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; van Veggel, F.C.J.M. Synthesis of nanoparticles, their biocompatibility, and toxicity behavior for biomedical applications. J. Mater. Chem. B 2013, 1, 5186–5200. [Google Scholar] [CrossRef]
- Flower, N.A.; Brabu, B.; Revathy, M.; Gopalakrishnan, C.; Raja, S.V.; Murugan, S.S.; Kumaravel, T.S. Characterization of synthesized silver nanoparticles and assessment of its genotoxicity potentials using the alkaline comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 742, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Sung, J.H.; Ji, J.H.; Song, K.S.; Lee, J.H.; Kang, C.S.; Yu, I.J. In vivo genotoxicity of silver nanoparticles after 90-day silver nanoparticle inhalation exposure. Saf. Health Work 2011, 2, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ji, J.H.; Song, K.S.; Lee, J.H.; Choi, K.H.; Lee, S.H.; Yu, I.J. Acute inhalation toxicity of silver nanoparticles. Toxicol. Ind. Health 2011, 27, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Munger, M.A.; Radwanski, P.; Hadlock, G.C.; Stoddard, G.; Shaaban, A.; Falconer, J.; Grainger, D.W.; Deering-Rice, C.E. In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cristobal, L.F.; Martinez-Castanon, G.A.; Loyola-Rodriguez, J.P.; Patino-Marin, N.; Reyes-Macias, J.F.; Vargas-Morales, J.M.; Ruiz, F. Toxicity, distribution, and accumulation of silver nanoparticles in Wistar rats. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Travan, A.; Pelillo, C.; Donati, I.; Marsich, E.; Benincasa, M.; Scarpa, T.; Semeraro, S.; Turco, G.; Gennaro, R.; Paoletti, S. Non-cytotoxic silver nanoparticle-polysaccharide nanocomposites with antimicrobial activity. Biomacromolecules 2009, 10, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Cheng, C.; Ma, Y.L.; Zhao, C.S. Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J. Mater. Sci. Mater. Med. 2010, 21, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Toshima, H.; Yonezawa, T.; Kawai, K.; Narushima, T.; Kaga, M.; Endo, K. Responses of raw264.7 macrophages to water-dispersible gold and silver nanoparticles stabilized by metal-carbon sigma-bonds. J. Biomed. Mater. Res. A 2013, 102, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.M.; Gueorguieva, M.; Lees, M.R.; McGarvey, D.J.; Hoskins, C. Physical stability, biocompatibility and potential use of hybrid iron oxide-gold nanoparticles as drug carriers. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Gamucci, O.; Bertero, A.; Gagliardi, M.; Bardi, G. Biomedical Nanoparticles: Overview of Their Surface Immune-Compatibility. Coatings 2014, 4, 139–159. [Google Scholar] [CrossRef]
- Fabian, E.; Landsiedel, R.; Ma-Hock, L.; Wiench, K.; Wohlleben, W.; van Ravenzwaay, B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch. Toxicol. 2008, 82. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Miller, M.L.; Di Pasqua, A.J. Biocompatibility of Mesoporous Silica Nanoparticles? Comm. Inorg. Chem. 2016, 36. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Aziz, S.A.B.; Ismail, I.; Tamilselvan, S.; Alitheen, N.B.; Swamy, M.K.; Rao, B.P.C. Synthesis, Characterization and in vitro evaluation of manganese ferrite (MnFe2O4) nanoparticles for their biocompatibility with murine breast cancer cells (4T1). Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Tomitaka, A.; Jeun, M.; Bae, S.; Takemura, Y. Evaluation of magnetic and thermal properties of ferrite nanoparticles for biomedical applications. J. Magnet. 2011, 16, 164–168. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Fontana, L.; Bergamaschi, A. Toxicological effects of titanium dioxide nanoparticles: A review of in vitro mammalian studies. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 481–508. [Google Scholar] [PubMed]
- Khanna, L.; Verma, N.K. Synthesis, characterization and in vitro cytotoxicity study of calcium ferrite nanoparticles. Mater. Sci. Semiconduct. Process. 2013, 16, 1842–1848. [Google Scholar] [CrossRef]
- Sosnik, A.; Carcaboso, A.M.; Glisoni, R.J.; Moretton, M.A.; Chiappetta, D.A. New old challenges in tuberculosis: Potentially effective nanotechnologies in drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Sarfati, G.; Dvir, T.; Elkabets, M.; Apte, R.N.; Cohen, S. Targeting of polymeric nanoparticles to lung metastases by surface-attachment of YIGSR peptide from laminin. Biomaterials 2011, 32, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Labhasetwar, V.; Song, C.; Levy, R.J. Nanoparticle drug delivery system for restenosis. Adv. Drug Deliv. Rev. 1997, 24, 63–85. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, K.G.; Stanley, A.M.; Aponte-Carro, J.C.; de Jésus, P.; Costantino, H.R.; Bosques, C.J.; Griebenow, K. Non-aqueous encapsulation of excipient-stabilized spray-freeze dried BSA into poly(lactide-co-glycolide) microspheres results in release of native protein. J. Control. Release 2001, 76, 199–208. [Google Scholar] [CrossRef]
- Kim, D.; El-Shall, H.; Dennis, D.; Morey, T. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf. B Biointerfaces 2005, 40, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.C.; Allémann, E.; de Jaeghere, F.; Doelker, E.; Gurny, R. Biodegradable nanoparticles from sustained release formulations to improved site specific drug delivery. J. Control. Release 1996, 39, 339–350. [Google Scholar] [CrossRef]
- Tobío, M.; Sánchez, A.; Vila, A.; Soriano, I.; Evora, C.; Vila-Jato, J.L.; Alonso, M.J. The role of PEG on the stability in digestive fluids and in vivo fate of PEGPLA nanoparticles following oral administration. Colloids Surf. B Biointerfaces 2000, 18, 315–323. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Nevozhay, D.; Kañska, U.; Budzyñska, R.; Boratyñski, J. Current status of research on conjugates and related drug delivery systems in the treatment of cancer and other diseases (Polish). Postepy Hig. Med. Dosw. 2007, 61, 350–360. [Google Scholar]
- Wilczewska, A.Z.; Katarzyna Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Steiniger, S.C.; Kreuter, J.; Khalansky, A.S.; Skidan, I.N.; Bobruskin, A.I.; Smirnova, Z.S.; Severin, S.E.; Uhl, R.; Kock, M.; Geiger, K.D.; et al. Chemotherapy of glioblastoma in rats using doxorubicinloaded nanoparticles. Int. J. Cancer 2004, 109, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.P.; Kopelman, R. Alexa Fluor 488 as an iron sensing molecule and its application in PEBBLE nanosensors. Analyst 2005, 130, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.R.; Bachmann, M.H.; Wang, Q.; Kasper, R.; Contag, C.H. PEGPLA/PLGA Nanoparticles for in vivo RNAi Delivery; NSTI Nano Tech.: Santa Clara, CA, USA, 2007. [Google Scholar]
- Tan, W.B.; Jiang, S.; Zhang, Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials 2007, 28, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.; Kohler, N.; Zhang, M. Self-Assembled Coatings on Individual Monodisperse Magnetite Nanoparticles for Efficient Intracellular Uptake. Biomed. Microdev. 2004, 6, 33–40. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, Z.; Afroz, H. Prospects and applications of nanobiotechnology: A medical perspective. J. Nanobiotechnol. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Wang, N.S. Nanoparticles in Biomedical Applications and Their Safety Concerns. In Biomedical Engineering—From Theory to Applications; Fazel-Rezai, R., Ed.; InTech Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health effects—Pros and Cons. Environ. Health Perspect. 2006, 114, 1818–1825. [Google Scholar] [CrossRef] [PubMed]


| Type of Nanoparticles | Mode of Action | Susceptible Microbes | References |
|---|---|---|---|
| Silver (Ag) nanoparticles | Interfere with the electron transport chain and transfer of energy through the membrane. Inhibit DNA replication and respiratory chain in bacteria and fungi. | Methicillin-resistant Staphylococcus aureus, Staphylococcus epidermidis. Vancomycin-resistant Enterococcus faecium and Klebsiella pneumoniae | [31,57,65] |
| Magnesium oxide (MgO) nanoparticles | Formation of reactive oxygen species (ROS), lipid peroxidation, electrostatic interaction, alkaline effect. | S. aureus, E. coli, Bacillus megaterium, Bacillus subtilis | [66,67] |
| Titanium dioxide (TiO2) nanoparticles | Formation of superoxide radicals, ROS, and site-specific DNA damage. | E. coli, S. aureus, and also against fungi | [28,31,68,69] |
| Zinc oxide (ZnO) nanoparticles | Hydrogen peroxide generated on the surface of ZnO penetrates the bacterial cells and effectively inhibits growth. Zn2+ ions released from the nanoparticles damage the cell membrane and interact with intracellular components. | E. coli, Listeria monocytogenes, Salmonella, and S. aureus | [70,71,72,73,74] |
| Gold (Au) nanoparticles | Generate holes in the cell wall. Bind to the DNA and inhibit the transcription process. | Methicillin-resistant S. aureus | [75,76,77,78] |
| Copper oxide (CuO) nanoparticles | Reduce bacteria at the cell wall. Disrupt the biochemical processes inside bacterial cells. | B. subtilis, S. aureus, and E. coli | [79,80,81,82] |
| Iron-containing nanoparticles | Through ROS-generated oxidative stress. ROS, superoxide radicals (O2−), singlet oxygen (1O2), hydroxyl radicals (OH−), and hydrogen peroxide (H2O2). | S. aureus, S. epidermidis, and E. coli. | [83] |
| Aluminum (Al) nanoparticles | Disrupt cell walls through ROS. | E. coli | [82,84] |
| Bismuth (Bi) nanoparticles | Alter the Krebs cycle, and amino acid and nucleotide metabolism. | Multiple-antibiotic resistant Helicobacter pylori | [85,86] |
| Carbon-based nanoparticles | Severe damage to the bacterial membrane, physical interaction, inhibition of energy metabolism, and impairment of the respiratory chain. | E. coli, Salmonella enteric, E. faecium, Streptococcus spp., Shewanella oneidensis, Acinetobacter baumannii, Burkholderia cepacia, Yersinia pestis, and K. pneumonia | [87,88,89,90,91] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. https://doi.org/10.3390/molecules21070836
Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules. 2016; 21(7):836. https://doi.org/10.3390/molecules21070836
Chicago/Turabian StyleRudramurthy, Gudepalya Renukaiah, Mallappa Kumara Swamy, Uma Rani Sinniah, and Ali Ghasemzadeh. 2016. "Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes" Molecules 21, no. 7: 836. https://doi.org/10.3390/molecules21070836
APA StyleRudramurthy, G. R., Swamy, M. K., Sinniah, U. R., & Ghasemzadeh, A. (2016). Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules, 21(7), 836. https://doi.org/10.3390/molecules21070836