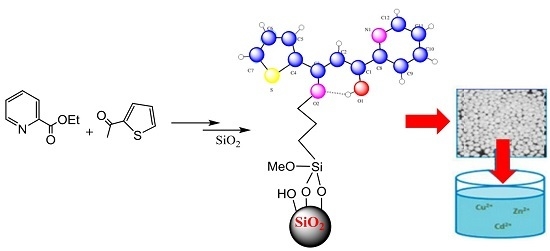
β-Keto-enol Tethered Pyridine and Thiophene: Synthesis, Crystal Structure Determination and Its Organic Immobilization on Silica for Efficient Solid-Liquid Extraction of Heavy Metals
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. X-ray Crystal Structure Description
2.3. Immobilization on Silica
2.3.1. Linker Synthesis
2.3.2. Characterization
2.3.3. Solid-Liquid Adsorption of Metal Ions
Effect of pH and Stirring Time
Adsorption Isotherms
Thermodynamics Adsorption
Competitive Adsorption
Comparison with Alternative Adsorbents
3. Materials and Methods
3.1. General Information
3.2. Procedure for the Synthesis of β-Keto-enol Heterocycle
3.3. Synthesis of 3-Aminopropylsilica (SiNH2)
3.4. Synthesis of Pyridine-enol-imine-thiophene-Substituted Silica (SiNTh-Py)
3.5. Batch Method
3.6. X-ray Diffraction Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okamura, H.; Hirayama, N.; Morita, K.; Shimojo, K.; Naganawa, H. Synergistic effect of 18-crown-6 derivatives on chelate extraction of lanthanoids(III) into an ionic liquid with 2-thenoyltrifluoroacetone. Anal. Sic. 2010, 26, 607–611. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lipparad, S.J. In vitro anticancer activity of cis-diammineplatinum(II) complexes with β-diketonate leaving group ligands. J. Med. Chem. 2012, 55, 5326–5336. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.F.; Shen, Z.H.; Shi, Y.; He, Q.; Xia, Q.C. Synthesis, characterization, crystal structure, and biological activity of the copper complex. Russ. J. Coord. Chem. 2010, 36, 458–462. [Google Scholar] [CrossRef]
- Almeida, J.C.; Marzano, I.M.; Silva de Paula, F.C.; Pivatto, M.; Lopes, N.P.; de Souza, P.C.; Pavan, F.R.; Forminga, A.L.B.; Pereira-Maia, E.C.; Guerra, W. Complexes of platinum and palladium with β-diketones and DMSO: Synthesis, characterization, molecular modeling, and biological studies. J. Mol. Struct. 2014, 1075, 370–376. [Google Scholar] [CrossRef]
- El-Sonbati, A.Z.; Diab, M.A.; Belal, A.A.; Morgan, S.M. Supramolecular structure and spectral studies on mixed-ligand complexes derived from β-diketone with azodye rhodanine derivatives. Spectrochim. Acta A 2012, 99, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Marchetti, F.; Drozdov, A. β-Diketones and related ligands. Compr. Coord. Chem. II 2003, 1, 97–115. [Google Scholar]
- Aromi, G.; Gamez, P.; Reedijk, J. Poly β-diketones: Prime ligands to generate supramolecular metalloclusters. Coord. Chem. Rev. 2008, 252, 964–989. [Google Scholar] [CrossRef]
- Sheikh, J.; Juneja, H.; Ingle, V.; Ali, P.; BenHadda, T. Synthesis and in vitro biology of Co(II), Ni(II), Cu(II) and Zinc(II) complexes of functionalized β-diketone bearing energy buried potential antibacterial and antiviral O,O pharmacophore sites. J. Saudi Chim. Soc. 2013, 17, 269–276. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, K. Rare Earth Coordination Chemistry; John Wiley & Sons (Asia) Pte Ltd.: 2 Clementi Loop, Singapore, 2010; pp. 41–85. [Google Scholar]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Thames Polytechnic: London, UK, 1989. [Google Scholar]
- Przeszlakowski, S.; Wydra, H. Extraction of nickel, cobalt and other metals (Cu, Zn, Fe(III)) with a commercial β-diketone extractant. Hydrometallurgy 1982, 8, 49–64. [Google Scholar] [CrossRef]
- Nakashima, K.; Maruyama, T.; Kubota, F.; Goto, M. Metal extraction from water and organic solvents into fluorous solvents by fluorinated beta-diketone and its application to the colorimetric analysis of metal ions. Anal. Sci. 2009, 25, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, J.; Rao, G.N. Solvent extraction of metals with a commercial fluorinated β-diketone (LIX51) extractant. Inorg. Anal. 1988, 100, 455–457. [Google Scholar]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Revel, B.; Zaghrioui, M. Quantitative removal of Zn(II) from aqueous solution and natural water using new silica-immobilized ketoenol-pyridine receptor. J. Environ. Chem. Eng. 2015, 3, 1769–1778. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; El Massaoudi, M.; Bacquet, M.; Degoutin, S.; Revel, B.; Mabkhot, Y.N. Thermodynamics and Kinetics of Heavy Metals Adsorption on Silica Particles Chemically Modified by Conjugated β-Ketoenol Furan. J. Chem. Eng. Data 2015, 60, 2915–2925. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, Y.; Bacquet, M.; Degoutin, S.; Mabkhot, Y.N.; Garcia, Y. An inorganic-organic hybrid material made of a silica-immobilized Schiff base receptor and its preliminary use in heavy metal removal. ACS Adv. 2016, 6, 34212–34218. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Zaghrioui, M. New adsorbent material based on nitrothiophene-functionalized silica particles for aqueous heavy metals removal. J. Sulfur Chem. 2016, 37, 296–306. [Google Scholar] [CrossRef]
- Radi, S.; Attayibat, A.; El Massaoudi, M.; Bacquet, M.; Jodeh, S.; Warad, I.; Al-Showiman, S.S.; Mabkhot, Y.N. C,N-Bipyrazole Receptor Grafted onto a Porous Silica Surface as a Novel Adsorbent Based Polymer Hybrid. Talanta 2015, 143, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tighadouini, S.; Radi, S.; Bacquet, M.; Dacquin, J.-P.; Mabkhot, Y.N.; Jodeh, S.; Warad, I.; Zaghrioui, M. Synthesis of 1-(Furan-2-yl) imine Functionalized Silica as a Chelating Sorbent and its Preliminary Use in Metal Ion Adsorption. Sep. Sci. Technol. 2015, 50, 710–717. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Feron, O.; Riant, O.; Bouakka, M.; Benabbes, R.; Mabkhot, Y.N. Synthesis of Novel β-Keto-enol Derivatives Tethered Pyrazole, Pyridine and Furan as New Potential Antifungal and Anti-Breast Cancer Agents. Molecules 2015, 20, 20186–20194. [Google Scholar] [CrossRef] [PubMed]
- Radi, S.; Tighadouini, S.; Ben Hadda, T.; Akkurt, M.; Özdemir, N.; Sirajuddin, M.; Mabkhot, Y.N. Crystal structure of (2Z)-3-hydroxy-1-(1,5-dimethyl-1Hpyrazol-3-yl)but-2-en-1-one. Z. Kristallogr. NCS 2016, 231, 617–618. [Google Scholar]
- Radi, S.; Tighadouini, S.; Eddike, D.; Tillard, M.; Mabkhot, Y.N. Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(4-methoxyphenyl)prop-2-en-1-one. Z. Kristallogr. NCS 2016. submitted. [Google Scholar]
- Radi, S.; Tighadouini, S.; Eddike, D.; Tillard, M.; Mabkhot, Y.N. Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-(4-ethoxyphenyl)-3-hydroxyprop-2-en-1-one. Z. Kristallogr. NCS 2016. submitted. [Google Scholar]
- Radi, S.; Tighadouini, S.; Eddike, D.; Tillard, M.; Mabkhot, Y.N. Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-phenylprop-2-en1–1-one. Z. Kristallogr. NCS 2016. submitted. [Google Scholar]
- Radi, S.; Tighadouini, S.; Eddike, D.; Tillard, M.; Mabkhot, Y.N. Crystal structure of (Z)-1-(1,5-dimethyl-1H-pyrazol-3-yl)-3-hydroxy-3-(p-toly)prop-2-en-1-one. Z. Kristallogr. NCS 2016. submitted. [Google Scholar]
- Qu, R.; Wang, M.; Sun, C.; Zhang, Y.; Ji, C.; Chen, H.; Meng, Y.; Yin, P. Chemical modification of silica-gel with hydroxyl-oramino-terminated polyamine for adsorption of Au(III). Appl. Surf. Sci. 2008, 255, 3361–3370. [Google Scholar] [CrossRef]
- Radi, S.; Basbas, N.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F. New amine-modified silicas: Synthesis, characterization and its use in the Cu(II) removalfrom aqueous solutions. Prog. Nanotechnol. Nanomater. 2013, 2, 108–116. [Google Scholar] [CrossRef]
- Lagergren, S. Theorie der sogenannten adsorption geloster stoffe, kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption process. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Sari, A.; Tuzen, M.; Uluözlü, Ö.D.; Soylak, M. Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochem. Eng. J. 2007, 37, 151–158. [Google Scholar] [CrossRef]
- Fan, T.; Liu, Y.; Feng, B.; Zeng, G.; Yang, C.; Zhou, M.; Zhou, H.; Tan, Z.; Wang, X. Biosorption of cadmium(II), zinc(II) and lead(II) by Penicillium simplicissimum: Isotherm, kinetics and thermodynamics. J. Hazard. Mater. 2008, 160, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Ucun, H.; Bayhan, Y.K.; Kaya, Y. Kinetic and thermodynamic studies of the biosorption of Cr(VI) by Pinus sylvestris Linn. J. Hazard. Mater. 2008, 153, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Pouilloux, H.; Hamad, H.; Saad, Z.; Kazpard, V. Divalent heavy metals adsorption onto different types of EDTA-modified mesoporous materials: Effectiveness and complexation rate. Micropor. Mesopor. Mater. 2015, 212, 125–136. [Google Scholar] [CrossRef]
- Parambadath, S.; Mathew, A.; Park, S.S.; Ha, C.S. Pentane-1,2-dicarboxylic acid functionalized spherical MCM-41: A simple and highly selective heterogeneous ligand for the adsorption of Fe3+ from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 1918–1927. [Google Scholar] [CrossRef]
- Mutneja, R.; Singh, R.; Kaur, V.; Wagler, J.; Felsc, S.; Kroke, E. Schiff base tailed silatranes for the fabrication of functionalized silica based magnetic nano-cores possessing active sites for the adsorption of copper ions. New J. Chem. 2016, 40, 1640–1648. [Google Scholar]
- Zhu, Z. Preparation and characterization of functionalized silica spheres for removal of Cu(II), Pb(II), Cr(VI) and Cd(II) from aqueous solutions. RSC Adv. 2015, 5, 28624–28632. [Google Scholar] [CrossRef]
- Wondracek, M.H.P.; Jorgetto, A.O.; Silva, A.C.P.; Ivassechen, J.D.R.; Schneider, J.F.; Saeki, M.J.; Pedrosa, V.A.; Yoshito, W.K.; Colauto, F.; Ortiz, W.A.; et al. Synthesis of mesoporous silica-coated magnetic nanoparticles modified with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazoleand its application as Cu(II) adsorbent from aqueous samples. Appl. Surf. Sci. 2016, 367, 533–541. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, V.D.S. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound: (2Z)-3-hydroxy-3-(pyridin-2-yl)-1-(thiophen-2-yl)prop-2-en-1-one and the material (SiNTh-Py) are available from the authors.









| CCDC Deposition Number | 1481979 |
| Molecular Formula | C12H9NO2S |
| Molecular Weight | 231.26 |
| Crystal System | orthorhombic |
| Space Group | P n a 21 |
| a (Å) | 15.2526 (9) |
| b (Å) | 18.3543 (10) |
| c (Å) | 3.8806 (3) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| V (Å3) | 1086.38 (11) |
| Z | 4 |
| Dcalc (g·cm−3) | 1.414 |
| Crystal Dimension (mm) | 0.35 × 0.13 × 0.12 |
| μ (mm−1) | 0.280 |
| Tmin/Tmax | 0.908/0.967 |
| Measured Reflections | 4873 |
| −17, 17 | |
| Indices Range (h, k, l) | −21, 19 |
| −5, 3 | |
| θ Limit (°) | 1.736–27.718 |
| Unique Reflections | 1801 |
| Observed Reflections (I > 2σ(I)) | 1402 |
| Parameters | 151 |
| Goodness of Fit on F2 | 1155 |
| R1, wR2 (I > 2σ(I)) | 0.0612, 0.1578 |
| Parameters | Metals | |||
|---|---|---|---|---|
| Cu(II) | Zn(II) | Cd(II) | Pb(II) | |
| qe(exp) (mg/g) | 104.12 | 98.90 | 72.02 | 65.54 |
| Pseudo-first-order | ||||
| qe (mg/g) | 19.78 | 33.28 | 10.07 | 22.02 |
| k1 (min−1) | 0.128 | 0.153 | 0.102 | 0.169 |
| R2 | 0.960 | 0.960 | 0.997 | 0.945 |
| Pseudo-second-order | ||||
| qe (mg/g) | 104.16 | 90.90 | 71.94 | 66.22 |
| k2 (g/mg·min) | 3.76 × 10−3 | 17.28 × 10−3 | 23.29 × 10−3 | 29.61 × 10−3 |
| R2 | 0.993 | 0.997 | 0.999 | 0.998 |
| Metal | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||
|---|---|---|---|---|---|---|
| q (mg/g) | KL (L/mg) | R2 | KF (mg/g) | n | R2 | |
| Cu(II) | 106.38 | 0.425 | 0.998 | 39.87 | 04.23 | 0.774 |
| Zn(II) | 96.15 | 0.138 | 0.995 | 16.74 | 02.47 | 0.923 |
| Cd(II) | 78.12 | 0.141 | 0.994 | 08.54 | 01.90 | 0.941 |
| Pb(II) | 67.56 | 0.328 | 0.998 | 26.58 | 04.59 | 0.829 |
| Metal | ΔH° (kJ·mol−1) | ΔS° (Jk−1·mol−1) | T (°C) ± 1 °C | ΔG° (kJ·mol−1) |
|---|---|---|---|---|
| Cu(II) | 19.55 | 70.23 | 25 | −1.45 |
| 35 | −2.15 | |||
| 45 | −2.89 | |||
| Zn(II) | 10.23 | 36.16 | 25 | −0.58 |
| 35 | −0.94 | |||
| 45 | −1.30 | |||
| Cd(II) | 09.27 | 31.45 | 25 | −0.13 |
| 35 | −0.44 | |||
| 45 | −0.76 | |||
| Pb(II) | 22.05 | 78.95 | 25 | −1.56 |
| 35 | −2.35 | |||
| 45 | −3.14 |
| Support: Silica Gel/Ligand | Ref. | Adsorption Capacity (mg/g) |
|---|---|---|
| ((Z)-3-hydroxy-3-(pyridine-2-yl)-1-(thiophen-2-yl)prop-2-en-1-on | This work | 104.12 |
| 1-(Furan-2-yl) imine | [19] | 77.48 |
| EDTA | [35] | 85.75 |
| Pentane-1,2-dicarboxylic acid | [36] | 38.00 |
| Schiff base tailed silatranes | [37] | 13.15 |
| Stearic acid | [38] | 63.00 |
| 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole | [39] | 05.02 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Dacquin, J.-P.; Eddike, D.; Tillard, M.; Mabkhot, Y.N. β-Keto-enol Tethered Pyridine and Thiophene: Synthesis, Crystal Structure Determination and Its Organic Immobilization on Silica for Efficient Solid-Liquid Extraction of Heavy Metals. Molecules 2016, 21, 888. https://doi.org/10.3390/molecules21070888
Radi S, Tighadouini S, Bacquet M, Degoutin S, Dacquin J-P, Eddike D, Tillard M, Mabkhot YN. β-Keto-enol Tethered Pyridine and Thiophene: Synthesis, Crystal Structure Determination and Its Organic Immobilization on Silica for Efficient Solid-Liquid Extraction of Heavy Metals. Molecules. 2016; 21(7):888. https://doi.org/10.3390/molecules21070888
Chicago/Turabian StyleRadi, Smaail, Said Tighadouini, Maryse Bacquet, Stephanie Degoutin, Jean-Philippe Dacquin, Driss Eddike, Monique Tillard, and Yahia N. Mabkhot. 2016. "β-Keto-enol Tethered Pyridine and Thiophene: Synthesis, Crystal Structure Determination and Its Organic Immobilization on Silica for Efficient Solid-Liquid Extraction of Heavy Metals" Molecules 21, no. 7: 888. https://doi.org/10.3390/molecules21070888
APA StyleRadi, S., Tighadouini, S., Bacquet, M., Degoutin, S., Dacquin, J. -P., Eddike, D., Tillard, M., & Mabkhot, Y. N. (2016). β-Keto-enol Tethered Pyridine and Thiophene: Synthesis, Crystal Structure Determination and Its Organic Immobilization on Silica for Efficient Solid-Liquid Extraction of Heavy Metals. Molecules, 21(7), 888. https://doi.org/10.3390/molecules21070888