In Vitro Reversible and Time-Dependent CYP450 Inhibition Profiles of Medicinal Herbal Plant Extracts Newbouldia laevis and Cassia abbreviata: Implications for Herb-Drug Interactions
Abstract
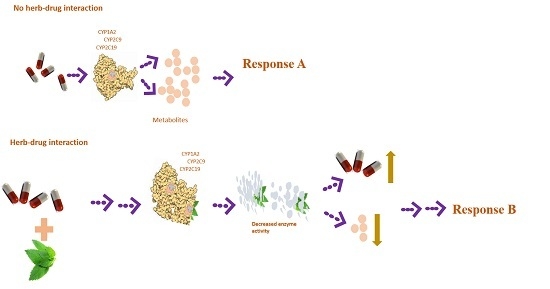
:1. Introduction
2. Results
2.1. Inhibition and TDI Potency
2.2. Kinetics of Inactivation
2.3. Phytofingerprinting by UPLC-MS
2.4. Prediction of in Vivo Herb–Drug Interaction for IC50
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Extraction
4.3. CYP450 Inhibition
4.4. Determination of Time-Dependent Inhibition (TDI) Potency
4.5. Estimation of Kinetics of Inactivation
4.6. UPLS-MS Analysis and Relative Quantification of Phenolic Compounds
4.7. Data Analysis
4.7.1. IC50 Determination
4.7.2. Inactivation Kinetics
4.7.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ritchie, M.R. Use of herbal supplements and nutritional supplements in the UK: What do we know about their pattern of usage? Proc. Nutr. Soc. 2007, 66, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, A.; Egan, B.; de Klein, S.; Dima, L.; Maggi, M.F.; Isoniemi, M.; Ribas-Barba, L.; Raats, M.M.; Meissner, E.M.; Badea, M.; et al. Usage of plant food supplements across six European countries: Findings from the PlantLIBRA consumer survey. PLoS ONE 2014, 9, e92265. [Google Scholar]
- Bandaranayake, W.M. Quality Control, Screening, Toxicity, and Regulation of Herbal Drugs. In Modern Phytomedicine: Turning Medicinal Plants into Drugs; Ahmed, I., Aqil, F., Owais, M., Eds.; John Wiley-VCH Verlag GmbH & Co. KGaA: Weinhelm, Germany, 2006. [Google Scholar]
- Thomford, N.E.; Dzobo, K.; Chopera, D.; Wonkam, A.; Skelton, M.; Blackhurst, D.; Chirikure, S.; Dandara, C. Pharmacogenomics Implications of Using Herbal Medicinal Plants on African Populations in Health Transition. Pharmaceuticals (Basel) 2015, 8, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.K. Association of Ginkgo biloba with intracerebral hemorrhage. Neurology 1998, 50, 1933–1994. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, S.; Zanetti, O.; Binetti, G.; Trabucchi, M.; Frisoni, G.B. Coma in a patient with Alzheimer′s disease taking low dose trazodone and gingko biloba. J. Neurol. Neurosurg. Psychiatry 2000, 68, 679–680. [Google Scholar] [CrossRef]
- Lantz, M.S. Reversible dementia as a result of herbal supplements and medications. Clin. Geriatr. 2008, 16, 145–149. [Google Scholar]
- Tsai, H.-H.; Lin, H.-W.; Lu, Y.-H.; Chen, Y.-L.; Mahady, G.B. A review of potential harmful interactions between anticoagulant/antiplatelet agents and Chinese herbal medicines. PLoS ONE 2013, 8, e64255. [Google Scholar] [CrossRef] [PubMed]
- Ainooson, G.K. Antinociceptive Effects of Newbouldia laevis (P. Beauv.) Stem Bark Extract in a Rat Model. Pharmacogn. Mag. 2009, 5, 49–54. [Google Scholar]
- Kolawole, O.T.; Akanji, M.A. Inhibitory effect of leaf extract of Newbouldia laevis on the metabolic activities of alpha-glucosidase and alpha-amylase. Bangladesh J. Pharmacol. 2013, 8, 371–377. [Google Scholar] [CrossRef]
- Mojeremane, W.; Legwaila, G.M.; Mogotsi, K.K.; Tshwenyane, S.O. Monepenepe (Cassia abbriviata): A Medicinal Plant in Botswana. Int. J. Bot. 2005, 1, 108–110. [Google Scholar]
- Mongala, N.I.; Mafoko, B.J. Cassia abbreviata Oliv. A review of its ethnomedicinal uses, toxicology, phytochemistry, possible propagation techniques and Pharmacology. African J. Pharm. Pharmacol. 2013, 7, 2901–2906. [Google Scholar]
- Klos, M.; van de Venter, M.; Milne, P.J.; Traore, H.N.; Meyer, D.; Oosthuizen, V. In vitro anti-HIV activity of five selected South African medicinal plant extracts. J. Ethnopharmacol. 2009, 124, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: The past, present and future. Trends Pharmacol. Sci. 2004, 25, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fugh-berman, A. Herb-drug interactions. Lancet 2000, 134–138. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A. A review and study based on assessment of clinical case reports in literature. Indian J. Pharmacol. 2007, 39, 129–139. [Google Scholar]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Wilkinson, G.R. Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 2005, 352, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Ogu, C.C.; Maxa, J.L. Drug interactions due to cytochrome P450. Proc. Bayl. Univ. Med. Cent. 2000, 13, 421–423. [Google Scholar] [PubMed]
- Berry, L.M.; Zhao, Z. An examination of IC50 and IC50-shift experiments in assessing time-dependent inhibition of CYP3A4, CYP2D6 and CYP2C9 in human liver microsomes. Drug Metab. Lett. 2008, 2, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Manda, V.K.; Dale, O.R.; Awortwe, C.; Ali, Z.; Khan, I.; Walker, L.; Khan, S.I. Evaluation of drug interaction potential of Labisia pumila (Kacip Fatimah) and its constituents. Front. Pharmacol. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Awortwe, C.; Bouic, P.J.; Masimirembwa, C.M.; Rosenkranz, B. Inhibition of Major Drug Metabolizing CYPs by Common Herbal Medicines used by HIV/AIDS Patients in Africa—Implications for Herb-Drug Interactions. Drug Metab. Lett. 2013, 7, 83–95. [Google Scholar] [CrossRef]
- Thomford, N.E.; Awortwe, C.; Dzobo, K.; Adu, F.; Chopera, D.; Wonkam, A.; Skelton, M.; Blackhurst, D.; Dandara, C. Inhibition of CYP2B6 by Medicinal Plant Extracts: Implication for Use of Efavirenz and Nevirapine-Based Highly Active Anti-Retroviral Therapy (HAART) in Resource-Limited Settings. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Rai, S.; Bhattacharya, S.; Wahile, A.; Saha, B.P. Marker analysis of polyherbal formulation, Triphala—A well known Indian traditional medicine. Indian J. Tradit. Knowl. 2008, 7, 379–383. [Google Scholar]
- Tripathi, M.; Sikarwar, R.L.S.; Tiwari, A.; Dwivedi, N. Pharmacognostical identification of ingredients in Laghulai curna: An Ayurvedic compound formulation. Indian J. Tradit. Knowl. 2015, 14, 531–536. [Google Scholar]
- Bjornsson, T.D.; Callaghan, J.T.; Einolf, H.J.; Fischer, V.; Gan, L.; Grimm, S.; Kao, J.; King, S.P.; Miwa, G.; Ni, L.; et al. The conduct of in vitro and in vivo drug-drug interaction studies: A PhRMA perspective. J. Clin. Pharmacol. 2003, 43, 443–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.D.; Zhao, P.; Huang, S.-M. Predicting drug-drug interactions: An FDA perspective. AAPS J. 2009, 11, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F.; Zhou, Z.-W.; Li, C.-G.; Chen, X.; Yu, X.; Xue, C.C.; Herington, A. Identification of drugs that interact with herbs in drug development. Drug Discov. Today 2007, 12, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, Z.; Upton, R.; Craker, L.; Bechtel, D.; Gurley, B.J.; Parks, C. Botanical Safety, 2nd ed.; CRC Press: New York, NY, USA, 2013. [Google Scholar]
- Riley, R.J.; Grime, K.; Weaver, R. Time-dependent CYP inhibition. Expert Opin. Drug Metab. Toxicol. 2007, 3, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.W.; Einolf, H.J.; Hall, S.D.; He, K.; Lim, H.K.; Ling, K.H.J.; Lu, C.; Nomeir, A.; Seibert, E.; Skordos, K.W.; et al. The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: A perspective of the Pharmaceutical Research and Manufacturers of America. Drug Metab. Dispos. 2009, 37, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Walsky, R.L.; Venkatakrishnan, K. Mechanism-based inactivation of human cytochrome p450 enzymes and the prediction of drug-drug interactions. Drug Metab. Dispos. 2007, 35, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Fasinu, P.S.; Gutmann, H.; Schiller, H.; James, A.D.; Bouic, P.J.; Rosenkranz, B. The potential of sutherlandia frutescens for herb-drug interaction. Drug Metab. Dispos. 2013, 41, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Awortwe, C.; Manda, V.K.; Avonto, C.; Khan, S.I.; Khan, I.A.; Walker, L.A.; Bouic, P.J.; Rosenkranz, B. In Vitro Evaluation of Reversible and Time-Dependent Inhibitory Effects of Kalanchoe crenata on CYP2C19 and CYP3A4 Activities. Drug Metab. Lett. 2015, 9, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Clauson, K.A.; Santamarina, M.L.; Rutledge, J.C. Clinically relevant safety issues associated with St. John′s wort product labels. BMC Complement. Altern. Med. 2008, 8, 42. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 3175–3255. [Google Scholar]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Baer-Dubowska, W.; Szaefer, H.; Krajka-Kuzniak, V. Inhibition of murine hepatic cytochrome P450 activities by natural and synthetic phenolic compounds. Xenobiotica 1998, 28, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, T.M.; Kumar, R.M.; Agrawal, A.; Dubey, G.P.; Ilango, K. Comparative inhibitory potential of selected dietary bioactive polyphenols, phytosterols on CYP3A4 and CYP2D6 with fluorometric high-throughput screening. J. Food Sci. Technol. 2014, 52, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Krippendorff, B.-F.; Neuhaus, R.; Lienau, P.; Reichel, A.; Huisinga, W. Mechanism-based inhibition: Deriving KI and kinact directly from time-dependent IC50 values. J. Biomol. Screen 2009, 14, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.










| Peak | Rt (min) | [M − H]− | Proposed Formula | Error (ppm) | MS/MS Fragmentation | Proposed Compound |
|---|---|---|---|---|---|---|
| 1 | 1.30 | 181.0718 | C6H14O6 | 3.3 | 43, 73, 89, 101, 109 | d-mannitol |
| 2 | 1.60 | 341.1082 | C12H22O11 | −0.6 | 161, 179, 341 | Sucrose |
| 3 | 7.86 | 431.1177 | C18H24O12 | −3.0 | 137, 149, 178 | 6-β-epiacetylscandoside |
| 4 | 8.10 | 403.1237 | C17H24O11 | −0.7 | 271 | 6-β-hydroxygeniposide |
| 5 | 8.45 | 417.1392 | C18H26O11 | −1.2 | 109, 167, 195 | |
| 6 | 8.32 | 461.13 | C19H25O13 | 1.5 | 152, 167, 329 | Sibricose A3 |
| 7 | 9.35 | 491.1425 | C38H20O | −2.2 | 356, 466, 421, 323, 334, 266, 298, 304 | |
| 8 | 10.29 | 289.0713 | C15H14O6 | 0.3 | 179, 188, 205, 245 | Catechin |
| 9 | 12.11 | 435.1274 | C21H24O10 | −3.9 | 289, 313, 342, 311, 393 | Epiafzelechin-3-o-β-d-glucopyranoside |
| 10 | 12.73 | 537.1798 | C22H34O15 | −3.9 | ||
| 11 | 13.48 | 561.1395 | C30H26O11 | −0.4 | 289, 273 | Epicatechin-(4β→8) epiafzelechin |
| 12 | 14.37 | 273.0765 | C15H14O5 | 0.7 | 271, 289 | (Epi)-Afzelechin |
| 13 | 16.06 | 545.1439 | C30H26O10 | −1.7 | Guibourtinidol-(4α→8) epicatechin | |
| 14 | 17.01 | 529.1502 | C30H26O9 | 0.6 | Guibourtinidol-(4α→8) epiafzelechin | |
| 15 | 21.80 | 801.2195 | C45H38O14 | 1.7 | ||
| 16 | 22.19 | 785.2243 | C45H38O13 | 1.1 | Cassiaflavan-(4β→8) epiafzelechin | |
| 17 | 22.41 | 513.1546 | C30H26O8 | −0.6 | Cassiaflavan-(4α→6) epiafzelechin |
| Peak | Rt (min) | [M − H]− | Proposed Formula | Error (ppm) | MS/MS Fragmentation | Proposed Compound |
|---|---|---|---|---|---|---|
| 1 | 2.23 | 191.0196 | C6H8O7 | 2.1 | 111, 127, 129 | Citric acid |
| 2 | 5.60 | 191.0558 | C7H12O6 | 1.0 | 71, 101, 173 | Quinic acid |
| 3 | 6.60 | 349.1143 | C14H22O11 | 2.3 | 440, 518, 591, 600 | |
| 4 | 7.68 | 401.1087 | C17H22O11 | 0.7 | 321, 341, 382 | 10-Dehydrogardenoside |
| 5 | 7.90 | 609.2037 | C25H38O17 | 1.0 | 303, 371, 475, 554, 623 | |
| 6 | 8.20 | 625.1984 | C25H38O18 | 0.6 | 359, 499, 515, 593, 661 | |
| 7 | 10.63 | 619.1869 | C26H36O17 | –0.8 | 179, 191, 283, 383 | |
| 8 | 10.95 | 353.0876 | C16H18O9 | 0.8 | 179, 191 | Chlorogenic acid |
| 9 | 11.73 | 679.2097 | C28H40O19 | 1.6 | 609, 661 | 4-caffeoylquinic acid |
| 10 | 12.73 | 675.1899 | C32H36O16 | –3.9 | 173 | Elloramycin F |
| 11 | 13.00 | 337.0923 | C16H18O8 | –0.9 | 119, 163 | 3-p-coumaroylquinic acid |
| 12 | 13.90 | 415.1611 | C19H28O10 | 1.7 | Aragoside | |
| 13 | 15.00 | 773.2352 | C34H46O20 | 0.5 | 135, 147, 161, 179, 417, 452, 591, 619 | Newbouldiside A |
| 14 | 15.91 | 291.1088 | C12H20O8 | 2.7 | 105, 135, 147,161 | |
| 15 | 16.97 | 461.0713 | C21H18O12 | –1.5 | 285, 345, 461 | Luteolin 7-O-glucuronide |
| 16 | 17.58 | 755.2393 | C34H44O19 | –0.8 | 461, 593 | Luteoside B |
| 17 | 17.94 | 197.1176 | C11H18O3 | –1.0 | 173, 179, 187 | |
| 18 | 19.06 | 445.0776 | C21H18O11 | 1.1 | 269, 271 | Apigenin 7-O-β-glucuronide |
| 19 | 20.14 | 399.1649 | C19H28O9 | –1.5 | ||
| 20 | 21.17 | 245.0456 | C13H10O5 | 2.0 | 133, 161, 179, 217, 234 | Pimpinellin |
| 21 | 21.80 | 709.2343 | C33H42O17 | –0.1 | 222, 253, 275, 315, 335, 337 | |
| 22 | 22.88 | 285.0399 | C15H10O6 | 0.0 | 133, 161, 175, 191, 199, 217 | Luteolin |
| 23 | 23.22 | 207.0658 | C11H12O4 | 0.5 | 179 | Ferrulic acid methyl ester |
| Herbal Extracts | % Yield | Recommended Herbal | Putative GIT | Estimated Bioavailable |
|---|---|---|---|---|
| Dose (Single; mg) | Concentration (µg/mL) | Concentration (µg/mL) | ||
| Newbouldia laevis | 14.66 | 200 | 800 | 117.28 |
| Cassia abbreviata | 12.47 | 200 | 800 | 99.76 |
| Herbal Extracts | Inhibitor | IC50 (µg/mL) | Risk of HDI in the Gut * | Ki | Predicted % Inhibition |
|---|---|---|---|---|---|
| Concentration (µg/mL) | |||||
| CYP1A2 | |||||
| Newbouldia laevis | 117.28 | 13.87 | likely | 4.86 | 96.02 |
| Cassia abbreviata | 99.76 | 3.35 | likely | 2.84 | 97.23 |
| CYP2C9 | |||||
| Newbouldia laevis | 117.28 | 17.92 | likely | 5.98 | 95.15 |
| Cassia abbreviata | 99.76 | 6.22 | likely | 1.55 | 98.47 |
| CYP2C19 | |||||
| Newbouldia laevis | 117.28 | 33.96 | likely | 1.58 | 98.67 |
| Cassia abbreviata | 99.76 | 1.27 | likely | 1.23 | 98.78 |
| Parameter | CYP1A2 | CYP2C9 | CYP2C19 |
|---|---|---|---|
| Substrate (µM) | 3 (EOMCC) | 10 (BOMCC) | 10 (EOMCC) |
| Enzyme (nM) | 5 | 10 | 5 |
| Standard inhibitor | α-naphthoflavone/furafylline | Sulphaphenazole | Miconazole |
| Phosphate buffer | 100 mM | 100 mM | 100 mM |
| Fluorescence filter | Ex: 405 nm/Em: 460 nm | Ex: 405 nm/Em:460 nm | Ex: 405 nm/Em: 460 nm |
| Reaction buffer (nM) | 200 (Buffer I) | 100 (Buffer II) | 100 (Buffer II) |
| Fluorescent product | 7-hydroxy-3-cyanocoumarin | 7-hydroxycoumarin | 7-hydroxy-3-cyanocoumarin |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomford, N.E.; Dzobo, K.; Chopera, D.; Wonkam, A.; Maroyi, A.; Blackhurst, D.; Dandara, C. In Vitro Reversible and Time-Dependent CYP450 Inhibition Profiles of Medicinal Herbal Plant Extracts Newbouldia laevis and Cassia abbreviata: Implications for Herb-Drug Interactions. Molecules 2016, 21, 891. https://doi.org/10.3390/molecules21070891
Thomford NE, Dzobo K, Chopera D, Wonkam A, Maroyi A, Blackhurst D, Dandara C. In Vitro Reversible and Time-Dependent CYP450 Inhibition Profiles of Medicinal Herbal Plant Extracts Newbouldia laevis and Cassia abbreviata: Implications for Herb-Drug Interactions. Molecules. 2016; 21(7):891. https://doi.org/10.3390/molecules21070891
Chicago/Turabian StyleThomford, Nicholas Ekow, Kevin Dzobo, Denis Chopera, Ambroise Wonkam, Alfred Maroyi, Dee Blackhurst, and Collet Dandara. 2016. "In Vitro Reversible and Time-Dependent CYP450 Inhibition Profiles of Medicinal Herbal Plant Extracts Newbouldia laevis and Cassia abbreviata: Implications for Herb-Drug Interactions" Molecules 21, no. 7: 891. https://doi.org/10.3390/molecules21070891
APA StyleThomford, N. E., Dzobo, K., Chopera, D., Wonkam, A., Maroyi, A., Blackhurst, D., & Dandara, C. (2016). In Vitro Reversible and Time-Dependent CYP450 Inhibition Profiles of Medicinal Herbal Plant Extracts Newbouldia laevis and Cassia abbreviata: Implications for Herb-Drug Interactions. Molecules, 21(7), 891. https://doi.org/10.3390/molecules21070891