Metabolic Responses of Bacterial Cells to Immobilization
Abstract
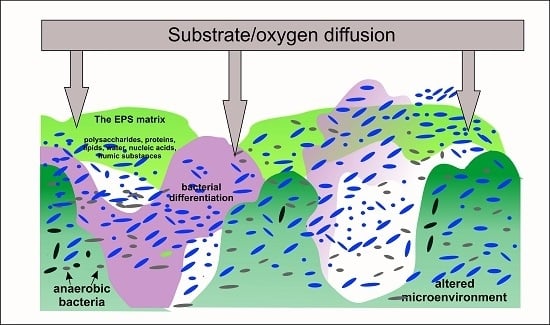
:1. Introduction
2. Conditions of Bacterial Cells Immobilization
3. Metabolic Responses to Immobilization
3.1. Growth Rate
3.2. Biocatalytic Efficiency/Changing Yields or New Metabolic Behaviour of Immobilized Cells
3.3. Biodegradation/Biotransformation Capacity of IC Systems
3.4. Nucleic Acids Content/Plasmid Stability
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mitra, A.; Mukhopadhyay, S. Biofilm mediated decontamination of pollutants from environments. AIMS Bioeng. 2016, 3, 44–59. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Costeron, J.W.; Marrie, T.J.; Cheng, K.-J. Phenomena of Bacterial Adhesion. In Bacterial Adhesion. Mechanisms and Physiological Significance, 1st ed.; Savage, D.C., Fletcher, M., Eds.; Plenum Press: New York, NY, USA, 1985; Volume 1, pp. 3–43. [Google Scholar]
- Van Loosdrecht, M.C.M.; Lyklema, J.; Norde, W.; Zehnder, A.J.B. Influence of interfaces on microbial activity. Microbiol. Rev. 1990, 54, 75–87. [Google Scholar] [PubMed]
- Costeron, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilm. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.E.; O’Toole, G.E. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Abee, T.; Kovacs, A.T.; Kuipers, O.P.; van der Veen, S. Biofilm formation and dispersal in Gram-positive bacteria. Curr. Opin. Biotechnol. 2011, 22, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Smet, C.; van Derlinden, E.; Mertens, L.; Noriega, E.; Van Impe, J.F. Effect of cell immobilization on the growth dynamics of Salmonella Typhimurium and Escherichia coli at suboptimal temperatures. Int. J. Food Microbiol. 2015, 208, 75–83. [Google Scholar] [CrossRef] [PubMed]
- ZoBell, Z.E. The effect of solid surfaces upon bacterial activity. J. Bacteriol. 1943, 46, 39–56. [Google Scholar] [PubMed]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Environmental applications of immobilized microbial cells: A review. J. Ind. Microbiol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- Junter, G.-A.; Coquet, L.; Vilain, S.; Jouenne, T. Immobilized-cell physiology: Current data and the potentialities of proteomics. Enzyme Microb. Technol. 2002, 31, 201–212. [Google Scholar] [CrossRef]
- Junter, G.-A.; Jouenne, T. Immobilized viable microbial cells: From the process to the proteome or the cart before the horse. Biotechnol. Adv. 2004, 22, 633–658. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.V.; Prakasham, R.S. Microbial fermentations with immobilized cells. Curr. Sci. 1999, 77, 87–100. [Google Scholar]
- Martins, S.C.S.; Martins, C.M.; Fiuza, L.M.C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418. [Google Scholar]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of microbes for bioremediation of crude oil polluted environments: A mini review. Open Microbiol. J. 2015, 9, 48–54. [Google Scholar] [PubMed]
- Morikawa, M. Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J. Biosci. Bioeng. 2006, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Sanjust, E. Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and supports materials. Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Hattori, T.; Hattori, R. The physical environment in soil microbiology: An attempt to extend principles of microbiology to soil microorganisms. CRC Crit. Rev. Microbiol. 1976, 4, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Bogino, P.C.; de las Mercedes Oliva, M.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell Infect. Microbiol. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Ghotaslou, R.; Salahi, B. Effects of oxygen on in vitro biofilm formation and antimicrobial resistance of Pseudomonas aeruginosa. Pharm. Sci. 2013, 19, 96–99. [Google Scholar]
- Stewart, E.J.; Ganesan, M.; Solomon, M.J. Artificial biofilm establish the role of matrix interactions in staphylococcal biofilm assembly and disassembly. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Auguet, O.; Pijuan, M.; Batista, J.; Borrego, C.M.; Gutierrez, O. Changes of microbial biofilms communities during colonization of sewer systems. Appl. Environ. Microbiol. 2015, 81, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Chu, F.; Kolter, R.; Losick, R. Bistability and biofilm formation in Bacillus subtilis. Mol. Microbiol. 2008, 67, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.J.; Dutilh, B.B.; Skennerton, C.T.; Fukushima, T.; Hastie, M.L.; Gorman, J.J.; Tyson, G.W.; Bond, P.L. Metagenomic and metaproteomic analyses of Accumulibacter phosphatis-enriched floccular and granular biofilms. Environ. Microbiol. 2016, 18, 273–287. [Google Scholar] [CrossRef] [PubMed]
- More, T.; Yadav, J.S.S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zheng, L.; Sun, D. Functions and behaviors of activated sludge extracellular polymeric substances (EPS): A promising environmental interest. J. Environ. Sci. 2006, 18, 420–427. [Google Scholar]
- Wingender, J.; Neu, T.R.; Flemming, H.-C. Microbial Extracellular Polymeric Substances: Characterization, Structure and Function, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 2–13. [Google Scholar]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef] [PubMed]
- Kolwzan, B. Analysis of Biofilms—Their formation and functioning. Ochr. Sr. 2011, 33, 3–14. (In Polish) [Google Scholar]
- Cao, B.; Shi, L.; Brown, R.N.; Xiong, Y.; Fredrickson, J.K.; Romine, M.F.; Marshall, M.J.; Lipton, M.S.; Beyenal, B. Extracellular polymeric substances from Shewannela sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ. Microbiol. 2011, 13, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Ahmed, B.; Kennedy, D.W.; Wang, Z.; Shi, L.; Marshall, M.J.; Fredrickson, J.K.; Isern, N.G.; Majors, P.D.; Beyenal, H. Contribution of Extracellular Polymeric Substances from Shewanella sp. HRCR-1 Biofilm to U(VI) Immobilization. Environ. Sci. Technol. 2011, 45, 5483–5490. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Leang, C.; Franks, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Specific location of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ. Microbiol. Rep. 2011, 3, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fang, H.H.P. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Marshall, K.C.; Stout, R.; Mitchell, R. Mechanisms of the initial events in the sorption of marine bacteria to surfaces. J. Gen. Microbiol. 1971, 68, 337–348. [Google Scholar] [CrossRef]
- Smith, M.R.; de Haan, A.; de Bont, J.A.M. The effect of calcium alginate entrapment on the physiology of Mycobacterium sp. strain E3. Appl. Microbiol. Biot. 1993, 38, 642–648. [Google Scholar] [CrossRef]
- Shreve, G.S.; Vogel, T.M. Comparison of substrate utilization and growth kinetics between immobilized and suspended Pseudomonas cells. Biotechnol. Bioeng. 1993, 41, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, R.J.; Brocklehurst, T.F.; Wilson, D.R.; Wilson, P.D.G. The effects of cell immobilization, pH and sucrose on the growth of Listeria monocytogenes Scott A at 10 °C. Food Microbiol. 2003, 20, 97–103. [Google Scholar] [CrossRef]
- Antwi, M.; Geeraerd, A.H.; Jennè, K.M.; Bernaerts, K.; van Impe, J.F. Influence of a gel microstructure as modified by gelatin concentration on Listeria innocua growth. Innov. Food Sci. Emerg. Technol. 2006, 7, 124–131. [Google Scholar] [CrossRef]
- Hattori, R.; Hattori, T. Growth rate and molar yield of E. coli adsorbed on an anion exchange resin. J. Gen. Appl. Microbiol. 1981, 27, 287–298. [Google Scholar] [CrossRef]
- Diefenbach, R.; Keweloh, H.; Rehm, H.J. Fatty acid impurities in alginate influence the phenol tolerance of immobilized Escherichia coli. Appl. Microbiol. Biotechnol. 1992, 36, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, G.; Rajesh Babu, S.; Mahesh, K.P.O.; Murugesan, T. Adsorption and biodegradation of phenol by chitosan-immobilized Pseudomonas putida (NICM 2174). Bioprocess Eng. 2000, 22, 493–501. [Google Scholar] [CrossRef]
- Nielsen, M.-B.; Knudsen, G.M.; Danino-Appleton, V.; Olsen, J.E.; Thomsen, L.E. Comparison of heat stress responses of immobilized and planktonic Salmonella enterica serovar Typhimurium. Food Microbiol. 2013, 33, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, D.C.; Keevil, C.W.; Marsh, P.D.; Brown, C.M.; Wardell, J.N. Surface-associated growth. Philos. Trans. R. Soc. Lond. Ser. B 1982, 297, 517–532. [Google Scholar] [CrossRef]
- Keen, G.A.; Prosser, J.I. Interrelationship between pH and surface growth of Nitrobacter. Soil Biol. Biochem. 1987, 19, 665–672. [Google Scholar] [CrossRef]
- Wilson, N.G.; Bradley, G. A study of a bacterial immobilization substratum for use in the bioremediation of crude oil in a saltwater system. J. Appl. Microbiol. 1997, 83, 524–530. [Google Scholar] [CrossRef]
- Bonin, P.; Rontani, J.-F.; Bordenave, L. Metabolic differences between attached and free-living marine bacteria: Inadequacy of liquid cultures for describing in situ bacterial activity. FEMS Microbiol. Lett. 2001, 194, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Xu, Y.; Wang, H.-Y.; Zhao, J.; Gao, D.-M.; Li, F.-M.; Xing, B. Biodegradation of crude oil in contaminated soil by free and immobilized microorganisms. Pedosphere 2012, 22, 717–725. [Google Scholar] [CrossRef]
- Muyima, N.Y.O.; Cloete, T.E. Growth and phosphate uptake of immobilized Acinetobacter cells suspended in activated sludge mixed liquor. Water Res. 1995, 29, 2461–2466. [Google Scholar] [CrossRef]
- Lyngberg, O.K.; Thiagarajan, V.; Stemke, D.J.; Schottel, J.L.; Scriven, L.E.; Flickinger, M.C. A patch coating method for preparing biocatalytic films of Escherichia coli. Biotech. Bioeng. 1999, 62, 44–55. [Google Scholar] [CrossRef]
- Klingeberg, M.; Vorlop, K.D.; Antrakinian, C. Immobilization of anaerobic thermophilic bacteria for the production of cell-free thermostable α-amylases and pullulanases. Appl. Microbiol. Biotechnol. 1990, 33, 494–500. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.Y.; Hassan, M.A.; Said, W.Y.; El-Aassar, S.A. Effect of support materials on antibiotic MSW2000 production by Streptomyces violatus. J. Gen. Appl. Microbiol. 2003, 49, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-P.; Tseng, H.-Y.; Juang, R.-S. Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochem. 2003, 38, 1497–1507. [Google Scholar] [CrossRef]
- Scherer, P.; Sahm, H. Influence of sulphur-containing compounds on the growth of Methanosarcina barkeri in a defined medium. Eur. J. Appl. Microbiol. 1981, 12, 28–35. [Google Scholar] [CrossRef]
- Nasri, M.; Sayadi, S.; Barbotin, J.-N.; Dhulster, P.; Thomas, D. Influence of immobilization on the stability of pTG201 recombinant plasmid in some strains of Escherichia coli. Appl. Environ. Microbiol. 1987, 53, 740–744. [Google Scholar] [PubMed]
- Sayadi, S.; Nasri, M.; Barbotin, J.N.; Thomas, D. Effect of environmental growth conditions on plasmid stability, plasmid copy number, and catechol 2,3-dioxygenase activity in free and immobilized Escherichia coli cells. Biotech. Bioeng. 1989, 33, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.M.; Briasco, C.A.; Huang, J.; Geraats, B.G.M.; Barbotin, J.-N.; Thomas, D.; Luyben, K.C. Measurement of oxygen concentration gradients in gel-immobilized recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 1990, 33, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Barbotin, J.-N. Immobilization of recombinant bacteria: A strategy to improve plasmid stability. Ann. N. Y. Acad. Sci. 1994, 721, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, Z.; Cen, P.; Wong, W.K.R. Enhanced plasmid stability and production of hEGF by immobilized recombinant E. coli JM101. Biochem. Eng. J. 2006, 208, 215–219. [Google Scholar] [CrossRef]
- Melo, L.; Bott, T.R.; Fletcher, M.; Capdeville, B. Biofilms—Science and Technology, 1st ed.; Springer: Heidelberg, Germany, 1992; pp. 68–87. [Google Scholar]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Cunha, A.; Almeida, A.; Coelho, F.J.R.C.; Gomes, N.C.N.; Oliveira, V.; Santos, A.L. Bacterial extracellular enzymatic activity in globally changing aquatic ecosystems. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, 1st ed.; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; Volume 2, pp. 124–135. [Google Scholar]
- Hong, Y.; Brown, D.G. Variation in bacterial ATP level and proton motive force due to adhesion to adhesion to a solid surface. Appl. Environ. Microbiol. 2009, 75, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Al-Garni, S.M.; Ghanem, K.M.; Ibrahim, A.S. Biosorption of mercury by capsulated and slime-layer forming Gram-ve bacilli from an aqueous solution. Afr. J. Biotechnol. 2010, 9, 6413–6421. [Google Scholar]
- Jobby, R.; Jha, P.; Desai, N. Sinorhizobium, a potential organism for bioremediation of nickel. Int. J. Adv. Res. 2015, 3, 706–717. [Google Scholar]
- Zhao, W.; Zhang, Y.; Liu, Y.; Tan, M.; Weiting, Y.; Xie, H.; Ma, Y.; Sun, G.; Lv, G.; Zhao, S.; et al. Oxygen diffusivity in alginate/chitosan microcapsules. J. Chem. Technol. Biotechnol. 2013, 88, 449–455. [Google Scholar] [CrossRef]
- Goodman, A.E.; Marshall, K.C. Genetic responses of bacteria at surface. In Microbial Biofilm, 1st ed.; Lappin-Scott, H., Costeron, J.W., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 80–98. [Google Scholar]
- Tanaka, H.; Matsumura, M.; Veliky, I.A. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol. Bioeng. 1984, 26, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Golmohamadi, M.; Wilkinson, K.J. Diffusion of ions in a calcium alginate hydrogel-structure is the primary factors controlling diffusion. Carbohydr. Polym. 2013, 94, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2011, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Beyenal, H.; Tanyolaç, A. Simultaneous evaluation of effective diffusion coefficients of the substrates in a biofilm with a novel experimental method. Can. J. Chem. Eng. 1996, 74, 526–533. [Google Scholar] [CrossRef]
- Yu, J.; Pinder, K.L. Diffusion of lactose in acidogenic biofilms. Biotechnol. Bioeng. 1993, 41, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Boons, K.; van Derlinden, E.; Mertens, L.; Peeters, V.; van Impe, J.F. Effect of immobilization and salt concentration on the growth dynamics of Escherichia coli K-12 and Salmonella Typhimurium. J. Food Sci. 2013, 78, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ng, C.K.; Cohen, Y.; Cao, B. Cell growth and protein expression of Shewanella oneidensis in biofilms and hydrogel-entrapped cultures. Mol. BioSyst. 2014, 10, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Mattiason, B.; Hahn-Hägerdal, B. Microenvironmental effects on metabolic behaviour of immobilized cells a hyphotesis. Eur. J. Appl. Microbiol. Biotechnol. 1982, 16, 52–55. [Google Scholar] [CrossRef]
- Dijkstra, M.F.J.; Michorius, A.; Buwalda, H.; Panneman, H.J.; Winkelman, J.G.M.; Beenackers, A.A.C.M. Comparison of the efficiency of immobilized and suspended systems in photocatalytic degradation. Catal. Today 2001, 66, 487–494. [Google Scholar] [CrossRef]
- Esener, A.A.; Bol, G.; Kossan, N.W.F.; Roels, J.A. Effect of water activity on microbial growth. In Advances in Biotechnology I, 1st ed.; Moo-Young, M., Robinson, C.W., Vezina, C., Eds.; Pergamon Press: Toronto, ON, USA, 1981; p. 339. [Google Scholar]
- Branco, R.; Sousa, T.; Piedade, A.P.; Morais, P.V. Immobilization of Ochrobactrum tritici As5 on PTFE thin films for arsenite biofiltration. Chemosphere 2016, 146, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Ahalaya, N.; Ramachandra, T.V.; Kanamadi, R.D. Biosorption of heavy metals. Res. J. Chem. Environ. 2003, 7, 71–79. [Google Scholar]
- Zhang, Z.; Liu, S.; Miyoshi, T.; Matsuyama, N.J. Mitigated membrane fouling of anamox membrane bioreactor by microbiological bioreactor. Bioresour. Technol. 2016, 201, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Varma, V.; Yang, S.; Berenjian, A. Magnetic immobilization of Bacillus subtilis natto cells for menaquinone-7 fermentation. Appl. Microbiol. Biotechnol. 2016, 100, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Hattori, R.; Hattori, T. Adsorptive phenomena involving bacterial cells and an anion exchange resin. J. Gen. Appl. Microbiol. 1985, 32, 147–163. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, W.; Liu, J.; Caiyin, Q.; Qiao, J. Immobilization of Streptomyces thermotolerans 11432 on polyurethane foam to improve production of acetylisovaleryltylosin. J. Ind. Microbiol. Biot. 2015, 42, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Stormo, K.E.; Crawford, R.L. Preparation of encapsulated microbial cells for environmental application. Appl. Environ. Microbiol. 1992, 58, 727–730. [Google Scholar] [PubMed]
- Ishikawa, M.; Shigemori, K.; Hori, K. Application of the Adhesive Bacterionanofiber AtaA to a Novel Microbial Immobilization Method for the Production of Indigo as a Model Chemical. Biotechnol. Bioeng. 2013, 111, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Parente, E.; Ricciardi, A. Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl. Microbiol. Biotechnol. 1999, 52, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, S.; Gangadharan, D.; Nampoothiri, K.M.; Soccol, C.R.; Pandey, A. α-Amylases from microbial sources—An overview on recent developments. Food Technol. Biotechnol. 2006, 44, 173–184. [Google Scholar]
- Abdelwahed, N.A.M.; Danial, E.N.; El-Naggar, N.E.-A.; Mohamed, A.A. Optimization of alkaline protease production by Streptomyces ambofaciens in free and immobilized form. Am. J. Biochem. Biotechnol. 2014, 10, 1–13. [Google Scholar] [CrossRef]
- Okada, M.; Morinaga, H.; Nishijima, W. Activated carbon as a better habitat for water and wastewater treatment microorganisms. Water Sci. Technol. 2000, 42, 149–154. [Google Scholar]
- Sekaran, G.; Karthikeyan, S.; Gupta, V.K.; Boopathy, R.; Maharaja, P. Immobilization of Bacillus sp. in mesoporous activated carbon for degradation of sulphonated phenolic compound in wastewater. Mater. Sci. Eng. C 2013, 33, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B.; Galil, N.I. Biosorption of phenol and chlorophenols by acclimated residential biomass under bioremediation conditions in a sandy aquifer. Water Res. 2004, 38, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, I.; Hernández, P.; Lafuente, A.; Rodríguez-Llorente, I.D.; Caviedes, M.A.; Pajuelo, E. Self-bioremediation of cork-processing wastewaters by (chloro)phenol-degrading bacteria immobilised onto residual cork particles. Water Res. 2012, 46, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Qian, Y. Microbial degradation of 4-chlorophenol by microorganisms entrapped in carrageenan-chitosan gels. Chemosphere 1999, 38, 3109–3117. [Google Scholar] [PubMed]
- Cassidy, M.B.; Shaw, K.W.; Lee, H.; Trevors, J.T. Enhanced mineralization of pentachlorophenol by k-carrageenan encapsulated Pseudomonas sp. UG30. Appl. Microbiol. Biotechnol. 1997, 47, 108–113. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Shamaan, N.A.; Arif, N.M.; Koon, G.B.; Shukor, M.Y.A.; Syed, M.A. Enhanced phenol degradation by Acinetobacter sp. strain AQ5NOL 1. World J. Microbiol. Biotechnol. 2012, 28, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Pons, P.M.; Carmen Fuste, M. Uranium uptake by immobilized cells of Pseudomonas strain EPS 5028. Appl. Microbiol. Biotechnol. 1993, 39, 661–665. [Google Scholar] [CrossRef]
- Wong, P.K.; Lam, K.C.; So, C.M. Removal and recovery of Cu(II) from industrial effluent by immobilized cells of Pseudomonas putida II-11. Appl. Microbiol. Biotechnol. 1993, 39, 127–131. [Google Scholar] [CrossRef]
- Kanasawud, P.; Hjorleifsdottir, S.; Holst, O.; Mattiasson, B. Studies on immobilization of the thermophilic bacterium Thermus aquaticus YT-1 by entrapment in various matrices. Appl. Microbiol. Biotechnol. 1989, 31, 228–233. [Google Scholar] [CrossRef]
- Kuo, W.-Ch.; Shu, T.-Y. Biological pre-treatment of wastewater containing sulfate using anaerobic immobilized cells. J. Hazard. Mater. 2004, 113, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, H.; Shintani, M.; Omori, T. Divergence of mobile genetic elements involved in the distribution of xenobiotic-catabolic capacity. Appl. Microbiol. Biotechnol. 2004, 64, 154–174. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, B.; Altalhi, A.D. Degradative plasmid and heavy metal resistance plasmid naturally coexist in phenol and cyanide assimilating bacteria. Am. J. Biochem. Biotechnol. 2009, 5, 84–93. [Google Scholar]
- Bernal, V.; Gonzalez-Veracruz, M.; Canovas, M.; Iborra, J.L. Plasmid maintenance and physiology of a genetically engineered Escherichia coli strain during continuous l-carnitine production. Biotechnol. Lett. 2007, 29, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Dincbas, V.; Hortacsu, A.; Camurdan, A. Plasmid stability in immobilized mixed cultures of recombinant Escherichia coli. Biotechnol. Prog. 1993, 9, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hooijmans, C.M.; Briasco, C.A.; Geraats, B.G.M.; Lnyben, K.C.A.; Thomas, D.; Barbotin, J.-N. Effect of free-cell growth parameters on oxygen concentration profiles in gel-immobilized recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 1990, 33, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Zaghlou, T.; Mendawy, H.M.; El Assar, S.; Mostafa, M.H. Enhanced stability of the cloned Bacillus subtilis alkaline protease gene in alginate-immobilized B. subtilis cells. Enzyme Microb. Technol. 2002, 30, 862–866. [Google Scholar] [CrossRef]
| Support | Environmental Factors | Microbial Cell |
|---|---|---|
| Roughness | pH | EPS |
| Porosity | Oxygen concentration | Age of cells |
| Hydrophobicity | Temperature | Physiological state of cells |
| Superficial charge | Nutrient availability | Hydrophobicity |
| Toxicity | Flow velocity | Flagella, pilli |
| Type of functional groups | Cations/anions | Fimbriae, glycocalyx |
| Antimicrobial agents | Surface proteins | |
| Hydrodynamic forces | ||
| Adhesive forces | ||
| Rheology |
| Component of the EPS | Role | Typical Content | Ref. |
|---|---|---|---|
| Polysaccharides | Adhesion to abiotics/biotics surfaces, aggregation of bacteria, mechanical stability of biofilm, intracellular communication, retention of water, adsorption of organic and inorganic compounds, protective barrier to antibiotics, bacteriophages, predators, bacteriocins, ionic exchange, growth substrates | 40%–95% | [2,22,29,30,31,32,33] |
| Proteins | Adhesion, aggregation of bacteria, enzymatic activity, retention of water, tolerance to dry, sorption of organic and inorganic compounds, electron and donor acceptor, extracellular electron transfer mediated by matrix-associated proteins, ionic exchange, protective barrier | up to 60% | [2,22,29,30,31,32,33,34,35,36] |
| Nucleic acids | Adhesion, exchange of genetic information, export of cell components, horizontal gene transfer, growth substrates | up to 10% | [22,29,30,31,32,33] |
| Lipids | Flocculation, biosorption | up to 10% | [2,29,30,31,37] |
| Humic substances | Electron donors or acceptors | up to 30% | [29,37] |
| Metabolic Responses | Possible Explanation | Ref. |
|---|---|---|
| Increased growth rate | Nutrients adsorbed on surfaces | [4,8,10,11,12,13] |
| Support protection | ||
| Detoxification of inhibitors | ||
| pH buffering by ion exchange | ||
| Decreased growth rate | Mass transfer limitation | [8,40,41,42] |
| Diffusion limitation | ||
| Oxygen/nutrients gradient | ||
| Lack of nutrients adsorbed on surfaces | ||
| Increased adhesion of cells | Cell hydrophobicity | [4,21] |
| Higher productivity | Support protection | [1,10,11,12] |
| Increased tolerance to inhibitors and toxic compounds | ||
| Lower substrate affinity | Diffusion limitation | [4,43] |
| Altered pH | Differences between proton concentration at surface and in the bulk phase | [4,10] |
| Increased tolerance/resistance to inhibitors | Support protection | [10,16,33,44,45] |
| Detoxification of antibacterial substance | ||
| Alterations in composition and organization of cell wall and cell membrane | ||
| Higher protein-to-lipid ratio in membranes | ||
| Modification of membrane porins | ||
| Heat shock proteins (HSPs) and biosurfactants production | ||
| Point mutations | ||
| Horizontal gene transfer of resistance genes | ||
| Changes in protein production/different genes expression | Differences in types and ratio of proteins involved in biofilm formation, attachment of bacteria, amino acids and cofactors biosynthesis, adaption and protection of cells, variable genes expression within biofilms, planktonic and immobilized cells, increased invasiveness of immobilized cells | [1,11,12,46] |
| Bacterial Species | Immobilization Technique | Physiological Responses | Ref. |
|---|---|---|---|
| Nitrobacter sp. | Anion-exchange resin beads | Production of extracellular slime layer | [48] |
| Escherichia coli | Entrapment | Higher specific activity of enzyme; slower degradation of RNA | [53] |
| Clostridium thermosaccharolyticum | Entrapment in Ca-alginate | Higher specific activity and productivity of starch hydrolyzing enzymes | [54] |
| Marinobacter sp. | Porous glass beads | Increased metabolizing of c18-isoprenoid ketone; shorter generation times; higher CO2 production | [50] |
| Listeria monocytogenes | Gel Cassette System | Decreased growth rate | [4,41] |
| Escherichia coli | - | More oxidized glucose metabolites | |
| Streptomyces violatus | Sponge-cubes | Higher antibiotic production | [55] |
| Lactic acid bacteria | Ca-alginate, k-carrageenan beads | Increased lactic acid production | [13] |
| Acinetobacter sp. Pseudomonas putida | Gellan gum, chitosan, polyurethane | Phenol and chlorophenol biodegradation | [56] |
| Methanosarcina burkeri | Ca-alginate | Increased methane reduction rate | [57] |
| Escherichia coli | Polyacrylamide, polyvinyl alcohol, silica foam, glass and gelatin beads, agarose, Ca-alginate, k-carrageenan | Enhanced plasmid stability | [58,59,60,61,62] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic Responses of Bacterial Cells to Immobilization. Molecules 2016, 21, 958. https://doi.org/10.3390/molecules21070958
Żur J, Wojcieszyńska D, Guzik U. Metabolic Responses of Bacterial Cells to Immobilization. Molecules. 2016; 21(7):958. https://doi.org/10.3390/molecules21070958
Chicago/Turabian StyleŻur, Joanna, Danuta Wojcieszyńska, and Urszula Guzik. 2016. "Metabolic Responses of Bacterial Cells to Immobilization" Molecules 21, no. 7: 958. https://doi.org/10.3390/molecules21070958
APA StyleŻur, J., Wojcieszyńska, D., & Guzik, U. (2016). Metabolic Responses of Bacterial Cells to Immobilization. Molecules, 21(7), 958. https://doi.org/10.3390/molecules21070958