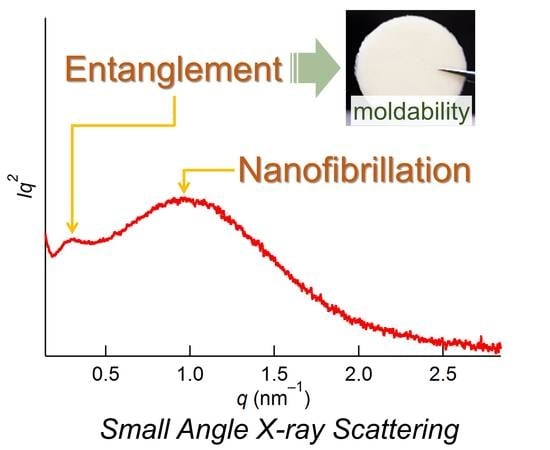
Nano-Structural Investigation on Cellulose Highly Dissolved in Ionic Liquid: A Small Angle X-ray Scattering Study
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Gupta, K.M.; Jiang, J. Cellulose dissolution and regeneration in ionic liquids: A computational perspective. Chem. Eng. Sci. 2015, 121, 180–189. [Google Scholar] [CrossRef]
- Yuan, X.; Cheng, G. From cellulose fibrils to single chains: Understanding cellulose dissolution in ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 31592–31607. [Google Scholar] [CrossRef] [PubMed]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Commun. 2006, 1271, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Youngs, T.G.A.; Holbrey, J.D.; Mullan, C.L.; Norman, S.E.; Lagunas, M.C.; D’Agostino, C.; Mantle, M.D.; Gladden, L.F.; Bowron, D.T.; Hardacre, C. Neutron diffraction, NMR and molecular dynamics study of glucose dissolved in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Chem. Sci. 2011, 2, 1594–1605. [Google Scholar] [CrossRef]
- Rabideau, B.D.; Agarwal, A.; Ismail, A.E. The role of the cation in the solvation of cellulose by imidazolium-based ionic liquids. J. Phys. Chem. B 2014, 118, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Hosomi, S.; Fujii, S.; Ninomiya, K.; Takahashi, K. Anion Bridging-Induced Structural Transformation of Cellulose Dissolved in Ionic Liquid. J. Phys. Chem. Lett. 2016, 7, 5156–5161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, X.; Simmons, B.; Singh, S. Theory, practice and prospects of X-ray and neutron scattering for lignocellulosic biomass characterization: Towards understanding biomass pretreatment. Energy Environ. Sci. 2015, 8, 436–455. [Google Scholar] [CrossRef]
- Nishiyama, Y. Structure and properties of the cellulose microfibril. J. Wood Sci. 2009, 55, 241–249. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gidley, M.J.; Gilbert, E.P. Application of X-ray and neutron small angle scattering techniques to study the hierarchical structure of plant cell walls: A review. Carbohydr. Polym. 2015, 125, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Doniach, S. Changes in biomolecular conformation seen by small angle X-ray scattering. Chem. Rev. 2001, 101, 1763–1778. [Google Scholar] [CrossRef] [PubMed]
- Le, K.A.; Rudaz, C.; Budtova, T. Phase diagram, solubility limit and hydrodynamic properties of cellulose in binary solvents with ionic liquid. Carbohydr. Polym. 2014, 105, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.M.; Khalfin, R.; Szekely, N.; Cohen, Y. True molecular solutions of natural cellulose in the binary ionic liquid-containing solvent mixtures. Carbohydr. Polym. 2014, 112, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, B. A new Guinier-Porod model. J. Appl. Crystallogr. 2010, 43, 716–719. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, K.; Andersson, S.; Torkkeli, M.; Knaapila, M.; Kotelnikova, N.; Serimaa, R. Structure of cellulose and microcrystalline cellulose from various wood species, cotton and flax studied by X-ray scattering. Cellulose 2009, 16, 999–1015. [Google Scholar] [CrossRef]
- Isogai, A. Wood nanocelluloses: Fundamentals and applications as new bio-based nanomaterials. J. Wood Sci. 2013, 59, 449–459. [Google Scholar] [CrossRef]
- Rabideau, B.D.; Agarwal, A.; Ismail, A.E. Observed Mechanism for the Breakup of Small Bundles of Cellulose Iα and Iβ in Ionic Liquids from Molecular Dynamics Simulations. J. Phys. Chem. B 2013, 117, 3469–3479. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Gross, A.S.; Chu, J.W. Dissecting force interactions in cellulose deconstruction reveals the required solvent versatility for overcoming biomass recalcitrance. J. Am. Chem. Soc. 2011, 133, 14033–14041. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sale, K.L.; Holmes, B.M.; Simmons, B.A.; Singh, S. Understanding the interactions of cellulose with ionic liquids: A molecular dynamics study. J. Phys. Chem. B 2010, 114, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhang, S.; Yao, Y.; Yao, X.; Xu, J.; Lu, X. Dissolving process of a cellulose bunch in ionic liquids: A molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 17894–17905. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Konishi, T. Structure analysis of regenerated cellulose hydrogels by small-angle and ultra-small-angle X-ray scattering. Phys. Rev. E 2000, 62, 727–733. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T.; Murata, K.; Hayashi, H.; Yoshitani, H. Investigation of the structure of cellulose in LiCl/DMAc solution and its gelation behavior by small-angle X-ray scattering measurements. Macromol. Biosci. 2006, 6, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Takada, A.; Kadokawa, J.-I. Fabrication and Characterization of Polysaccharide Ion Gels with Ionic Liquids and Their Further Conversion into Value-Added Sustainable Materials. Biomolecules 2015, 5, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Kunchornsup, W.; Sirivat, A. Effects of crosslinking ratio and aging time on properties of physical and chemical cellulose gels via 1-butyl-3-methylimidazolium chloride solvent. J. Sol-Gel Sci. Technol. 2010, 56, 19–26. [Google Scholar] [CrossRef]
- Debye, P.; Bueche, A.M. Scattering by an inhomogeneous solid. J. Appl. Phys. 1949, 20, 518–525. [Google Scholar] [CrossRef]
- Ornstein, L.S.; Zernike, F. Accidental deviations of density and opalescence at the critical point of a single substance. Proc. Acad. Sci. Amst. 1914, 17, 793–806. [Google Scholar]
- Sample Availability: Samples of the compounds are commercially available from the company mentioned in Section 3 Materials and Methods.






| Cellulose Concentration/mol % | Rg1/nm | d1 | s1 | Rg2/nm | d2 | s2 | ds |
|---|---|---|---|---|---|---|---|
| 30 | 7.9 ± 0.4 | 4.0 ± 0.0 | 0.0 ± 0.1 | n/a | n/a | n/a | n/a |
| 35 | 7.5 ± 0.2 | 3.8 ± 0.0 | 0.0 ± 0.1 | n/a | n/a | n/a | n/a |
| 40 | 7.1 ± 0.1 | 3.9 ± 0.0 | 0.0 ± 0.1 | n/a | n/a | n/a | n/a |
| 50 | 5.5 ± 1.5 | 3.0 ± 0.3 | 0.0 ± 0.8 | 1.6 ± 0.5 | 3.5 ± 0.4 | 0.0 ± 0.9 | 4.0 ± 1.5 |
| 60 | 5.9 ± 0.5 | 4.0 ± 0.0 | 0.0 ± 0.0 | 1.6 ± 0.0 | 4.0 ± 0.0 | 0.1 ± 0.1 | 4.0 ± 0.0 |
| 70 | 4.9 ± 0.2 | 3.6 ± 0.8 | 0.1 ± 0.1 | 1.6 ± 0.0 | 4.0 ± 0.0 | 0.1 ± 0.1 | 4.0 ± 0.0 |
| 80 | 4.7 ± 0.0 | 3.8 ± 0.4 | 0.1 ± 0.1 | 1.5 ± 0.1 | 4.0 ± 0.0 | 0.1 ± 0.1 | 4.0 ± 0.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, T.; Hosomi, S.; Fujii, S.; Ninomiya, K.; Takahashi, K. Nano-Structural Investigation on Cellulose Highly Dissolved in Ionic Liquid: A Small Angle X-ray Scattering Study. Molecules 2017, 22, 178. https://doi.org/10.3390/molecules22010178
Endo T, Hosomi S, Fujii S, Ninomiya K, Takahashi K. Nano-Structural Investigation on Cellulose Highly Dissolved in Ionic Liquid: A Small Angle X-ray Scattering Study. Molecules. 2017; 22(1):178. https://doi.org/10.3390/molecules22010178
Chicago/Turabian StyleEndo, Takatsugu, Shota Hosomi, Shunsuke Fujii, Kazuaki Ninomiya, and Kenji Takahashi. 2017. "Nano-Structural Investigation on Cellulose Highly Dissolved in Ionic Liquid: A Small Angle X-ray Scattering Study" Molecules 22, no. 1: 178. https://doi.org/10.3390/molecules22010178
APA StyleEndo, T., Hosomi, S., Fujii, S., Ninomiya, K., & Takahashi, K. (2017). Nano-Structural Investigation on Cellulose Highly Dissolved in Ionic Liquid: A Small Angle X-ray Scattering Study. Molecules, 22(1), 178. https://doi.org/10.3390/molecules22010178