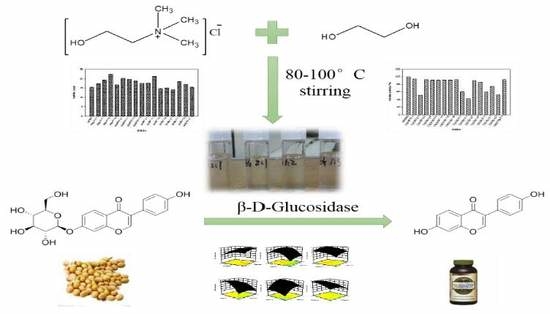
Highly Efficient Enzymatic Preparation of Daidzein in Deep Eutectic Solvents
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Screening of Optimum DES for the Enzymatic Reaction Based on Activity and Stability
2.2. The Influence of Different DESs as Additives on Enzymatic Activity and Stability
2.3. The Parameters of Enzymology Properties and Enzymatic Reaction Kinetics in DESs
2.4. Equilibrium Solubility of Daidzin in Different DESs
2.5. The Establishment of the Optimum Process Conditions
2.6. Preparation of Daidzein and Medium Reusability
2.7. The Superiority of ChCl/EG 2:1 Medium in Preparation of Daidzin
3. Experimental
3.1. Materials
3.2. Preparation of DESs and DESs-Containing Aqueous Solutions
3.3. Assays for β-d-Glucosidase Activity in DESs
3.4. Tests for β-d-Glucosidase Stability in DESs
3.5. Investigation of the Optimum Temperature and pH of β-d-Glucosidase in DESs
3.6. The Determination of Equilibrium Solubility of Daidzin in Different DESs
3.7. General Procedure for the Enzymatic Preparation of Daidzein
3.8. Optimization of the Enzymatic Reaction Conditions-Box-Behnken Experimental Design
3.9. Scale-Up Preparation of Daidzein and Medium Reusability
3.10. Controlled Trials Required by Traditional Hydrolysis Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Watanabe, S.; Uesugi, S.; Kikuchi, Y. Isoflavones for prevention of cancer, cardiovascular diseases, gynecological problems and possible immune potentiation. Biomed. Pharmacother. 2002, 56, 302–312. [Google Scholar] [CrossRef]
- Levis, S.; Strickman-Stein, N.; Doerge, D.R.; Krischer, J. Design and baseline characteristics of the Soy Phytoestrogens As Replacement Estrogen (SPARE) study—A clinical trial of the effects of soy isoflavones in menopausal women. Contemp. Clin. Trials 2010, 31, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Wei-Jie, Y. Effects of soy protein containing isoflavones in patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Nutr. 2016, 35, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chung, I.-M.; Kim, H.; Jung, M.Y. High resolution LC-ESI-TOF-mass spectrometry method for fast separation, identification, and quantification of 12 isoflavones in soybeans and soybean products. Food Chem. 2015, 176, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.C.; Morgan, M.R.A.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [PubMed]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [PubMed]
- Iovine, B.; Iannella, M.L.; Gasparri, F.; Giannini, V.; Monfrecola, G.; Bevilacqua, M.A. A Comparative Analysis of the Photo-Protective Effects of Soy Isoflavones in Their Aglycone and Glucoside Forms. Int. J. Mol. Sci. 2012, 13, 16444–16456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, S. The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and their Food Products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A. Factors affecting the bioavailability of soy isoflavones in humans. J. AOAC Int. 2006, 89, 1182–1188. [Google Scholar] [PubMed]
- Walsh, K.R.; Haak, S.J.; Bohn, T.; Tian, Q.; Schwartz, S.J.; Failla, M.L. Isoflavonoid glucosides are deconjugated and absorbed in the small intestine of human subjects with ileostomies. Am. J. Clin. Nutr. 2007, 85, 1050–1056. [Google Scholar] [PubMed]
- Liu, W.; Zhang, H.X.; Wu, Z.L.; Wang, Y.J.; Wang, L.J. Recovery of Isoflavone Aglycones from Soy Whey Wastewater Using Foam Fractionation and Acidic Hydrolysis. J. Agric. Food Chem. 2013, 61, 7366–7372. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Z.L.; Wang, Y.J.; Li, R.; Yin, N.N.; Jiang, J.X. Separation of isoflavone aglycones using chitosan microspheres from soy whey wastewater after foam fractionation and acidic hydrolysis. J. Ind. Eng. Chem. 2015, 25, 138–144. [Google Scholar] [CrossRef]
- Pei, X.; Zhao, J.; Cai, P.; Sun, W.; Ren, J.; Wu, Q.; Zhang, S.; Tian, C. Heterologous expression of a GH3 β-glucosidase from Neurospora crassa in Pichia pastoris with high purity and its application in the hydrolysis of soybean isoflavone glycosides. Protein Expr. Purif. 2016, 119, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Lee, Y.-S.; Fang, S.-J.; Park, D.-J.; Choi, Y.-L. Hydrolysis of isoflavone glycoside by immobilization of β-glucosidase on a chitosan-carbon in two-phase system. Int. J. Biol. Macromol. 2013, 61, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.; Yan, Q.; Jiang, Z.; Li, L. Hydrolysis of soybean isoflavone glycosides by a thermostable β-glucosidase from Paecilomyces thermophila. Food Chem. 2009, 115, 1247–1252. [Google Scholar] [CrossRef]
- Horii, K.; Adachi, T.; Matsuda, T.; Tanaka, T.; Sahara, H.; Shibasaki, S.; Ogino, C.; Hata, Y.; Ueda, M.; Kondo, A. Improvement of isoflavone aglycones production using β-glucosidase secretory produced in recombinant Aspergillus oryzae. J. Mol. Catal. B-Enzym. 2009, 59, 297–301. [Google Scholar] [CrossRef]
- Fang, W.; Yang, Y.; Zhang, X.; Yin, Q.; Zhang, X.; Wang, X.; Fang, Z.; Yazhong, X. Improve ethanol tolerance of β-glucosidase Bgl1A by semi-rational engineering for the hydrolysis of soybean isoflavone glycosides. J. Biotech. 2016, 227, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixturesElectronic supplementary information (ESI) available: Spectroscopic data. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuel 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Lindberg, D.; Revenga, M.D.L.F.; Widersten, M. Deep eutectic solvents (DESs) are viable cosolvents for enzyme-catalyzed epoxide hydrolysis. J. Biotechnol. 2010, 147, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Curko, N.; Tomasevic, M.; Kovacevic Ganic, K.; Radojcic Redovnikovic, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lee, P.I. Investigation on drug solubility enhancement using deep eutectic solvents and their derivatives. Int. J. Pharmaceut. 2016, 505, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-L.; Wu, B.-P.; Wen, Q.; Yang, T.-X.; Yang, Z. Deep eutectic solvents can be viable enzyme activators and stabilizers. J. Chem. Technol. Biotechnol. 2014, 89, 1975–1981. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Crittle, T.D. Choline-based deep eutectic solvents for enzymatic preparation of biodiesel from soybean oil. J. Mol. Catal. B-Enzym. 2013, 85–86, 243–247. [Google Scholar] [CrossRef]
- Maugeri, Z.; Leitner, W.; María, P.D.D. Chymotrypsin-Catalyzed Peptide Synthesis in Deep Eutectic Solvents. Eur. J. Org. Chem. 2013, 2013, 4223–4228. [Google Scholar] [CrossRef]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.H.; Zheng, Z.M.; Liu, H.; Wang, L.; Diao, J.S.; Wang, P.; Zhao, G.H. Purification and Characterization of a beta-Glucosidase from Aspergillus niger and Its Application in the Hydrolysis of Geniposide to Genipin. J. Microbiol. Biotechnol. 2014, 24, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-P.; Wen, Q.; Xu, H.; Yang, Z. Insights into the impact of deep eutectic solvents on horseradish peroxidase: Activity, stability and structure. J. Mol. Catal. B-Enzym. 2014, 101, 101–107. [Google Scholar] [CrossRef]
- Lehmann, C.; Sibilla, F.; Maugeri, Z.; Streit, W.R.; Domínguez de María, P.; Martinez, R.; Schwaneberg, U. Reengineering CelA2 cellulase for hydrolysis in aqueous solutions of deep eutectic solvents and concentrated seawater. Green Chem. 2012, 14, 2719. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Baréa, B.; Piombo, G.; Dubreucq, E.; Villeneuve, P. Evaluation of deep eutectic solvents as new media for Candida antarctica B lipase catalyzed reactions. Process Biochem. 2012, 47, 2081–2089. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Baréa, B.; Dubreucq, E.; Lortie, R.; Villeneuve, P. Evaluation of deep eutectic solvent-water binary mixtures for lipase-catalyzed lipophilization of phenolic acids. Green Chem. 2013, 15, 2275. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Kotogan, A.; Kecskemeti, A.; Szekeres, A.; Papp, T.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vagvolgyi, C.; Tako, M. Characterization of transesterification reactions by Mucoromycotina lipases in non-aqueous media. J. Mol. Catal. B-Enzym. 2016, 127, 47–55. [Google Scholar] [CrossRef]
- Weiz, G.; Braun, L.; Lopez, R.; de María, P.D.; Breccia, J.D. Enzymatic deglycosylation of flavonoids in deep eutectic solvents-aqueous mixtures: Paving the way for sustainable flavonoid chemistry. J. Mol. Catal. B-Enzym. 2016, 130, 70–73. [Google Scholar] [CrossRef]
- Yang, S.-L.; Duan, Z.-Q. Insight into enzymatic synthesis of phosphatidylserine in deep eutectic solvents. Catal. Commun. 2016, 82, 16–19. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Mazur, M.; Radosevic, K.; Redovnikovic, I.R. Baker’s yeast-mediated asymmetric reduction of ethyl 3-oxobutanoate in deep eutectic solvents. Process Biochem. 2015, 50, 1788–1792. [Google Scholar] [CrossRef]
- Nie, G.J.; Zheng, Z.M.; Jin, W.; Gong, G.H.; Wang, L. Development of a tannase biocatalyst based on bio-imprinting for the production of propyl gallate by transesterification in organic media. J. Mol. Catal. B-Enzym. 2012, 78, 32–37. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wang, G.; Zhang, J.; Hao, H.; Yin, Q. Solid-liquid equilibrium of sulbactam in pure solvents and binary solvent mixtures. Fluid Phase Equilib. 2014, 382, 197–204. [Google Scholar] [CrossRef]
- Negahdar, L.; Delidovich, I.; Palkovits, R. Aqueous-phase hydrolysis of cellulose and hemicelluloses over molecular acidic catalysts: Insights into the kinetics and reaction mechanism. Appl. Cata. B-Environ. 2016, 184, 285–298. [Google Scholar] [CrossRef]
- Dikshit, R.; Tallapragada, P. Partial Purification and Characterization of beta-glucosidase from Monascus sanguineus. Braz. Arch. Biol. Technol. 2015, 58, 185–191. [Google Scholar] [CrossRef]
- Sample Availability: Not available.






| Factors | Actual and Coded Levels Used for the Conditions | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| A = Reaction time (min) | 30 | 90 | 150 |
| B = Temperature (°C) | 45 | 55 | 65 |
| C = pH value | 3.0 | 5.0 | 7.0 |
| D = Enzyme loading (U) | 0.5 | 1.5 | 2.5 |
| Dependent variable (response) | Constrains | ||
| R = Conversion yield (%) | Maximize | ||
| Source | Sum of Square | df | Mean Square | F-Value | p-Value | Significance Level |
|---|---|---|---|---|---|---|
| Model | 26,090.77 | 14 | 1863.63 | 62.75 | <0.0001 | Sig |
| A-RT | 1184.05 | 1 | 1184.05 | 39.87 | <0.0001 | |
| B-Temp | 3717.12 | 1 | 3717.12 | 125.15 | <0.0001 | |
| C-pH | 7405.3 | 1 | 7405.3 | 249.32 | <0.0001 | |
| D-EL | 2745.19 | 1 | 2745.19 | 92.43 | <0.0001 | |
| AB | 0.16 | 1 | 0.16 | 0.005387 | 0.9425 | |
| AC | 51.12 | 1 | 51.12 | 1.72 | 0.2106 | |
| AD | 0.56 | 1 | 0.56 | 0.019 | 0.8925 | |
| BC | 481.8 | 1 | 481.8 | 16.22 | 0.0012 | |
| BD | 10.56 | 1 | 10.56 | 0.36 | 0.5605 | |
| CD | 128.82 | 1 | 128.82 | 4.34 | 0.0561 | |
| A2 | 541.98 | 1 | 541.98 | 18.25 | 0.0008 | |
| B2 | 1769.34 | 1 | 1769.34 | 59.57 | <0.0001 | |
| C2 | 9754.11 | 1 | 9754.11 | 328.4 | <0.0001 | |
| D2 | 836.1 | 1 | 836.1 | 28.15 | <0.0001 | |
| Residual | 415.82 | 14 | 29.7 | |||
| Lack of fit | 342.65 | 10 | 34.26 | 1.87 | 0.2858 | Not Sig |
| Pure Error | 73.17 | 4 | 18.29 | |||
| Cor Total | 26,506.59 | 28 | ||||
| R2 | 0.9843 | |||||
| Adj R2 | 0.9686 | |||||
| C.V. % | 9.74 | |||||
| Adj precision | 23.789 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.-B.; Zhang, L.-W. Highly Efficient Enzymatic Preparation of Daidzein in Deep Eutectic Solvents. Molecules 2017, 22, 186. https://doi.org/10.3390/molecules22010186
Cheng Q-B, Zhang L-W. Highly Efficient Enzymatic Preparation of Daidzein in Deep Eutectic Solvents. Molecules. 2017; 22(1):186. https://doi.org/10.3390/molecules22010186
Chicago/Turabian StyleCheng, Qi-Bin, and Li-Wei Zhang. 2017. "Highly Efficient Enzymatic Preparation of Daidzein in Deep Eutectic Solvents" Molecules 22, no. 1: 186. https://doi.org/10.3390/molecules22010186
APA StyleCheng, Q. -B., & Zhang, L. -W. (2017). Highly Efficient Enzymatic Preparation of Daidzein in Deep Eutectic Solvents. Molecules, 22(1), 186. https://doi.org/10.3390/molecules22010186