Alkylglycerol Derivatives, a New Class of Skin Penetration Modulators
Abstract
:1. Introduction
2. Results
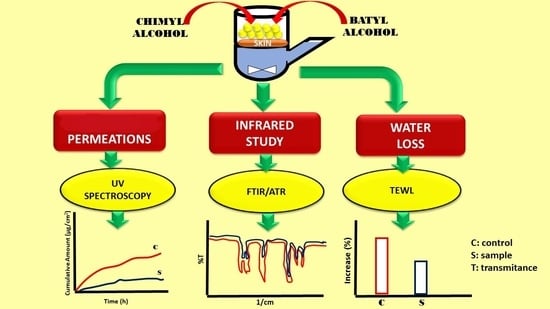
2.1. In Vitro Skin Permeation Studies
2.2. TEWL Measurement
2.3. ATR-FTIR Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. In Vitro Skin Permeation Studies
4.2.2. Transepidermal Water Loss (TEWL) Measurement
4.2.3. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
4.2.4. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AGs | alkylglycerols |
| ATR-FTIR | attenuated total reflectance-Fourier transform infrared |
| DI | diclofenac |
| ER | enhancement ratio |
| GLYAC | glycyrrhizic acid ammomium salt |
| NP | naproxen |
| PI | piroxicam |
| SC | stratum corneum |
| TEWL | transepidermal water loss |
References
- Bolzinger, M.-A.; Briancon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Alexander, A.; Dwivedi, S.; Ajazuddin; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J.; Peck, J.; Williams, D.; Pugh, W.; Allan, G. Mechanisms of action of skin penetration enhancers/retarders: Azone and analogues. Int. J. Pharm. 1996, 141, 17–25. [Google Scholar] [CrossRef]
- Kaushik, D.; Batheja, P.; Kilfoyle, B.; Rai, V.; Michniak-Kohn, B. Percutaneous permeation modifiers: Enhancement versus retardation. Expert Opin. Drug Deliv. 2008, 5, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.I.J.; McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Innovative Strategies for Enhancing Topical and Transdermal Drug Delivery. Open Drug Deliv. J. 2007, 1, 36–59. [Google Scholar] [CrossRef]
- Iannitti, T.; Palmieri, B. An update on the therapeutic role of alkylglycerols. Mar. Drugs 2010, 8, 2267–2300. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Sedeño, C.; Valdés, Y.; Mamposo, M.; Pérez, E. Éteres de glicerilo de origen marino: Un promotor de absorción. Rev. Cuba. Farm. 1993, 27, 123–133. [Google Scholar]
- Bilbao-Díaz, M.F.; Peña-Proenza, Y.; Gutierrez-Bueno, M.; Cabrera-Pérez, M.A.; Ducongé-Soler, J.; Linares-Cuéllas, G.M.; Fernández-Sánchez, E.M.; Valdés-Rodríguez, Y.C. Inferable analysis related to promotion of the absorption potentialities from 1-O-alkyl glycerol family. Rev. Cuba. Farm. 2009, 43, 1–9. [Google Scholar]
- Chun-Xiang, Z.; Qin, H. Determination of polyacid dissociation constants of glycyrrhizic acid. Indian J. Chem. 2008, 47A, 71–74. [Google Scholar]
- Boncheva, M.; Damien, F.; Normand, V. Molecular organization of the lipid matrix in intact Stratum corneum using ATR-FTIR spectroscopy. Biochim. Biophys. Acta 2008, 1778, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, R.; Flach, C.R.; Moore, D.J. Determination of molecular conformation and permeation in skin via IR spectroscopy, microscopy, and imaging. Biochim. Biophys. Acta 2006, 1758, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Prasch, T.; Knubel, G.; Schmidt-Fonk, K.; Ortanderl, S.; Nieveler, S.; Forster, T. Infrared spectroscopy of the skin: Influencing the stratum corneum with cosmetic products. Int. J. Cosmet. Sci. 2000, 22, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Escribano, E.; Calpena, A.C.; Queralt, J.; Obach, R.; Domenech, J. Assessment of diclofenac permeation with different formulations: Anti-inflammatory study of a selected formula. Eur. J. Pharm. Sci. 2003, 19, 203–210. [Google Scholar] [CrossRef]
- Manconi, M.; Caddeo, C.; Sinico, C.; Valenti, D.; Mostallino, M.C.; Biggio, G.; Fadda, A.M. Ex vivo skin delivery of diclofenac by transcutol containing liposomes and suggested mechanism of vesicle-skin interaction. Eur. J. Pharm. Biopharm. 2011, 78, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Michniak-Kohn, B. Percutaneous penetration modifiers and formulation effects: Thermal and spectral analyses. AAPS PharmaSciTech 2010, 11, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Nangia, A.; Patil, S.; Berner, B.; Boman, A.; Maibach, H. In vitro measurement of transepidermal water loss: A rapid alternative to tritiated water permeation for assessing skin barrier functions. Int. J. Pharm. 1998, 170, 33–40. [Google Scholar] [CrossRef]
- Elmahjoubi, E.; Frum, Y.; Eccleston, G.M.; Wilkinson, S.C.; Meidan, V.M. Transepidermal water loss for probing full-thickness skin barrier function: Correlation with tritiated water flux, sensitivity to punctures and diverse surfactant exposures. Toxicol. In Vitro 2009, 23, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Salgado, T.M.; Hadgraft, J.; Lane, M.E. The relationship between transepidermal water loss and skin permeability. Int. J. Pharm. 2010, 384, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ganem-Quintanar, A.; Lafforgue, C.; Falson-Rieg, F.; Buri, P. Evaluation of the transepidermal permeation of diethylene glycol monoethyl ether and skin water loss. Int. J. Pharm. 1997, 147, 165–171. [Google Scholar] [CrossRef]
- Hoppel, M.; Baurecht, D.; Holper, E.; Mahrhauser, D.; Valenta, C. Validation of the combined ATR-FTIR/tape stripping technique for monitoring the distribution of surfactants in the stratum corneum. Int. J. Pharm. 2014, 472, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.C.; Pagitsch, E.; Valenta, C. Comparison of ATR-FTIR spectra of porcine vaginal and buccal mucosa with ear skin and penetration analysis of drug and vehicle components into pig ear. Eur. J. Pharm. Sci. 2013, 50, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Bravo, H.; Quintanar-Guerrero, D.; Naik, A.; Kalia, N.Y.; Cornejo-Bravo, J.M.; Ganem-Quintanar, A. Effects of sucrose oleate and sucrose laureate on in vivo human stratum corneum permeability. Pharm. Res. 2003, 20, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Guy, R.H. Mechanisms of Transdermal Drug Delivery: Infrared Spectroscopy and Differential Scanning Calorimetric Investigation of the Stratum Corneum Barrier Function. In Mechanisms of Transdermal Drug Delivery; Potts, R.O., Guy, R.H., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 87–162. [Google Scholar]
- Casal, H.L.; Mantsch, H.H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim. Biophys. Acta 1984, 779, 381–401. [Google Scholar] [CrossRef]
- Imhof, R.E.; de Jesus, M.E.P.; Xiao, P.; Ciortea, L.I.; Berg, E.P. Closed chamber transepidermal water loss measurement: Microclimate, calibration and performance. Int. J. Cosmet. Sci. 2009, 31, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of alkylglycerol derivatives are not available from the authors.






| Name | Molecular Weight | pKa | Log P | Water Solubility |
|---|---|---|---|---|
| Diclofenac | 296.20 | 4.15 | 4.51 | 2.37 mg/L |
| Naproxen | 230.20 | 4.15 | 3.18 | 15.9 mg/L |
| Piroxicam | 331.34 | 6.30 | 3.06 | 23 mg/L |
| Glycyrrhizic acid ammonium salt | 822.94 | 3.98, 4.62, 5.17 1 | 2.80 | Freely sol in hot water |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Chávez, S.A.; Pérez-Carreto, L.Y.; Nava-Arzaluz, M.G.; Ganem-Rondero, A. Alkylglycerol Derivatives, a New Class of Skin Penetration Modulators. Molecules 2017, 22, 185. https://doi.org/10.3390/molecules22010185
Bernal-Chávez SA, Pérez-Carreto LY, Nava-Arzaluz MG, Ganem-Rondero A. Alkylglycerol Derivatives, a New Class of Skin Penetration Modulators. Molecules. 2017; 22(1):185. https://doi.org/10.3390/molecules22010185
Chicago/Turabian StyleBernal-Chávez, Sergio Alberto, Lilia Yazmín Pérez-Carreto, María Guadalupe Nava-Arzaluz, and Adriana Ganem-Rondero. 2017. "Alkylglycerol Derivatives, a New Class of Skin Penetration Modulators" Molecules 22, no. 1: 185. https://doi.org/10.3390/molecules22010185
APA StyleBernal-Chávez, S. A., Pérez-Carreto, L. Y., Nava-Arzaluz, M. G., & Ganem-Rondero, A. (2017). Alkylglycerol Derivatives, a New Class of Skin Penetration Modulators. Molecules, 22(1), 185. https://doi.org/10.3390/molecules22010185