Phloretin Exerts Anti-Tuberculosis Activity and Suppresses Lung Inflammation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anti-TB Activities of Phloretin
2.2. Effects of Phloretin on mRNA Expression of Inflammatory Cytokines in IFN-γ-Stimulated MRC-5 Cells and in LPS-Stimulated Dendritic Cells
2.3. Effects of phloretin on IFN-γ-Induced Protein Expression
2.4. Inflammatory Cytokine Levels in INF-γ-Stimulated MRC-5 Cells and LPS-Stimulated Dendritic Cells
2.5. TNF-α, IL-1β, and IL-6 Levels in LPS-Stimulated Mouse Lung Tissue
2.6. Cytotoxicity of Phloretin in Mammalian Cells and in LPS-Stimulated Mice
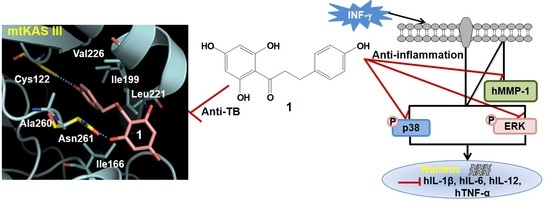
2.7. Phloretin–mtKASIII Binding Affinity and Docking Study of Phloretin with mtKASIII
3. Materials and Methods
3.1. Phloretin
3.2. Evaluation of Anti-TB Activity
3.3. Reverse Transcription-Polymerase Chain Reaction
3.4. Western Blot
3.5. Enzyme-Linked Immunosorbent Assay
3.6. In Vivo Mouse Model of LPS-Stimulated Lung Inflammation
3.7. Cytotoxicity of Phloretin against Mammalian Cells
3.8. Construction, Expression, and Purification of mtKASIII
3.9. Fluorescence Quenching between mtKASIII Protein and Phloretin
3.10. Docking Studies
3.11. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Hügel, H.M.; Jackson, N.; May, B.; Zhang, A.L.; Xue, C.C. Polyphenol protection and treatment of hypertension. Phytomedicine 2016, 23, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. Dietary polyphenols: Antioxidants or not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.J.R.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Escarpa, A.; González, M. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatogr. A 1998, 823, 331–337. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic Profiles in Eight Apple Cultivars Using High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Lommen, A.; Godejohann, M.; Venema, D.P.; Hollman, P.C.H.; Spraul, M. Application of Directly Coupled HPLC–NMR–MS to the Identification and Confirmation of Quercetin Glycosides and Phloretin Glycosides in Apple Peel. Anal. Chem. 2000, 72, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Bonarska-Kujawa, D.; Cyboran, S.; Oszmiański, J.; Kleszczyńska, H. Extracts from apple leaves and fruits as effective antioxidants. J. Med. Plants Res. 2011, 5, 2339–2347. [Google Scholar]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. Bioavailability of phloretin and phloridzin in rats. J. Nutr. 2001, 131, 3227–3230. [Google Scholar] [PubMed]
- Rezk, B.M.; Haenen, G.R.M.; van der Vijgh, W.J.; Bast, A. The antioxidant activity of phloretin: The disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 2002, 295, 9–13. [Google Scholar] [CrossRef]
- Stangl, V.; Lorenz, M.; Ludwig, A.; Grimbo, N.; Guether, C.; Sanad, W.; Ziemer, S.; Martus, P.; Baumann, G.; Stangl, K. The flavonoid phloretin suppresses stimulated expression of endothelial adhesion molecules and reduces activation of human platelets. J. Nutr. 2005, 135, 172–178. [Google Scholar] [PubMed]
- Chang, W.-T.; Huang, W.-C.; Liou, C.-J. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kim, J.-S.; Cha, S.B.; Kim, H.; Kwon, K.W.; Kim, S.J.; Han, S.J.; Choi, S.Y.; Cho, S.-N.; Park, J.-H.; et al. Mycobacterium tuberculosis Rv3628 drives Th1-type T cell immunity via TLR2-mediated activation of dendritic cells and displays vaccine potential against the hyper-virulent Beijing K strain. Oncotarget 2016, 7, 24962–24982. [Google Scholar] [CrossRef] [PubMed]
- Daffé, M.; Draper, P. The Envelope Layers of Mycobacteria with Reference to their Pathogenicity. Adv. Microb. Physiol. 1997, 39, 131–203. [Google Scholar]
- Barry, C.E.; Lee, R.E.; Mdluli, K.; Sampson, A.E.; Schroeder, B.G.; Slayden, R.A.; Yuan, Y. Mycolic acids: Structure, biosynthesis and physiological functions. Prog. Lipid Res. 1998, 37, 143–179. [Google Scholar] [CrossRef]
- Kremer, L.; Douglas, J.D.; Baulard, A.R.; Morehouse, C.; Guy, M.R.; Alland, D.; Dover, L.G.; Lakey, J.H.; Jacobs, W.R.; Brennan, P.J.; et al. Thiolactomycin and related analogues as novel anti-mycobacterial agents targeting KasA and KasB condensing enzymes in Mycobacterium tuberculosis. J. Biol. Chem. 2000, 275, 16857–16864. [Google Scholar] [CrossRef] [PubMed]
- Price, A.C.; Choi, K.H.; Heath, R.J.; Li, Z.; White, S.W.; Rock, C.O. Inhibition of beta-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin: Structure and mechanism. J. Biol. Chem. 2001, 276, 6551–6559. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Janson, C.A.; Konstantinidis, A.K.; Nwagwu, S.; Silverman, C.; Smith, W.W.; Khandekar, S.; Lonsdale, J.; Abdel-Meguid, S.S. Crystal structure of beta-ketoacyl-acyl carrier protein synthase III. A key condensing enzyme in bacterial fatty acid biosynthesis. J. Biol. Chem. 1999, 274, 36465–36471. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K. Control Mechanisms for Fatty Acid Synthesis in Mycobacterium Smegmatis. Adv. Enzymol. Relat. Areas Mol. Biol. 2006, 45, 1–84. [Google Scholar]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- White, S.W.; Zheng, J.; Zhang, Y.-M.; Rock, C.O. The Structural biology of Type II fatty acid biosynthesis. Annu. Rev. Biochem. 2005, 74, 791–831. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; Sridharan, S.; Kremer, L.; Lindenberg, S.; Dover, L.G.; Sacchettini, J.C.; Besra, G.S. Probing the mechanism of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthase III mtFabH: Factors influencing catalysis and substrate specificity. J. Biol. Chem. 2005, 280, 32539–32547. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Kremer, L.; Besra, G.S.; Rock, C.O. Identification and substrate specificity of beta-ketoacyl (acyl carrier protein) synthase III (mtFabH) from Mycobacterium tuberculosis. J. Biol. Chem. 2000, 275, 28201–28207. [Google Scholar] [PubMed]
- Scarsdale, J.N.; Kazanina, G.; He, X.; Reynolds, K.A.; Wright, H.T. Crystal structure of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthase III. J. Biol. Chem. 2001, 276, 20516–20522. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, W.; Li, R.-J.; Li, S. Synthesis of Potent Inhibitors of β-Ketoacyl-Acyl Carrier Protein Synthase III as Potential Antimicrobial Agents. Molecules 2012, 17, 4770–4781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-Y.; Jeong, K.-W.; Shin, S.; Lee, J.-U.; Kim, Y. Discovery of novel selective inhibitors of Staphylococcus aureus β-ketoacyl acyl carrier protein synthase III. Eur. J. Med. Chem. 2012, 47, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-W.; Lee, J.-Y.; Kang, D.-I.; Lee, J.-U.; Shin, S.Y.; Kim, Y. Screening of Flavonoids as Candidate Antibiotics against Enterococcus faecalis. J. Nat. Prod. 2009, 72, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Bilenki, L.; Gao, X.; Wang, S.; Yang, J.; Fan, Y.; Han, X.; Qiu, H.; Yang, X. Dendritic Cells from Mycobacteria-Infected Mice Inhibits Established Allergic Airway Inflammatory Responses to Ragweed via IL-10- and IL-12-Secreting Mechanisms. J. Immunol. 2010, 184, 7288–7296. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Minelli, L.; Rosignoli, P. Apple intake and cancer risk: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2016, 19, 2603–2617. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.-J.; Shen, J.; Jia, Y.-L.; Li, F.-F.; Ma, W.-J.; Shen, H.-J.; Shen, L.-L.; Lin, X.-X.; Zhang, L.-H.; Dong, X.-W.; et al. Apple polyphenol protects against cigarette smoke-induced acute lung injury. Nutrition 2013, 29, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Jnawali, H.N.; Jeon, D.; Jeong, M.-C.; Lee, E.; Jin, B.; Ryoo, S.; Yoo, J.; Jung, I.D.; Lee, S.J.; Park, Y.-M.; et al. Antituberculosis Activity of a Naturally Occurring Flavonoid, Isorhamnetin. J. Nat. Prod. 2016, 79, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Collart, M.A.; Belin, D.; Vassalli, J.D.; de Kossodo, S.; Vassalli, P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J. Exp. Med. 1986, 164, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Vila-del Sol, V.; Fresno, M. Involvement of TNF and NF-κB in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J. Immunol. 2005, 174, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Ford, C.B.; Coleman, M.T.; Myers, A.J.; Gawande, R.; Ioerger, T.; Sacchettini, J.; Fortune, S.M.; Flynn, J.L. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat. Med. 2013, 20, 75–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattila, J.T.; Ojo, O.O.; Kepka-Lenhart, D.; Marino, S.; Kim, J.H.; Eum, S.Y.; Via, L.E.; Barry, C.E.; Klein, E.; Kirschner, D.E.; et al. Microenvironments in Tuberculous Granulomas Are Delineated by Distinct Populations of Macrophage Subsets and Expression of Nitric Oxide Synthase and Arginase Isoforms. J. Immunol. 2013, 191, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Chan, J.; Lin, P.L. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011, 4, 271–278. [Google Scholar] [CrossRef]
- Mariotti, S.; Sargentini, V.; Pardini, M.; Giannoni, F.; De Spirito, M.; Gagliardi, M.C.; Greco, E.; Teloni, R.; Fraziano, M.; Nisini, R. Mycobacterium tuberculosis may escape helper T cell recognition by infecting human fibroblasts. Hum. Immunol. 2013, 74, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kang, J.-W.; Lee, D.H.; Kim, M.S.; Bak, Y.; Yang, Y.; Lee, H.G.; Hong, J.; Yoon, D.-Y. Interleukin-32α downregulates the activity of the B-cell CLL/lymphoma 6 protein by inhibiting protein kinase Cε-dependent SUMO-2 modification. Oncotarget 2014, 5, 8765–8777. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, J.-K.; Shin, S.; Jeong, K.-W.; Shin, A.; Lee, J.; Lee, D.G.; Hwang, J.-S.; Kim, Y. Insight into the antimicrobial activities of coprisin isolated from the dung beetle, Copris tripartitus, revealed by structure–activity relationships. Biochim. Biophys. Acta Biomembr. 2013, 1828, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jeong, K.-W.; Jnawali, H.; Shin, A.; Heo, Y.-S.; Kim, Y. Cytotoxic Activity of 3,6-Dihydroxyflavone in Human Cervical Cancer Cells and Its Therapeutic Effect on c-Jun N-Terminal Kinase Inhibition. Molecules 2014, 19, 13200–13211. [Google Scholar] [CrossRef] [PubMed]
- Jnawali, H.N.; Lee, E.; Jeong, K.-W.; Shin, A.; Heo, Y.-S.; Kim, Y. Anti-inflammatory Activity of Rhamnetin and a Model of Its Binding to c-Jun NH2-Terminal Kinase 1 and p38 MAPK. J. Nat. Prod. 2014, 77, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, K.-W.; Jin, B.; Ryu, K.-S.; Kim, E.-H.; Ahn, J.-H.; Kim, Y. Structural and Dynamic Features of Cold-Shock Proteins of Listeria monocytogenes, a Psychrophilic Bacterium. Biochemistry 2013, 52, 2492–2504. [Google Scholar] [CrossRef] [PubMed]
- Musayev, F.; Sachdeva, S.; Neel Scarsdale, J.; Reynolds, K.A.; Wright, H.T. Crystal Structure of a Substrate Complex of Mycobacterium tuberculosis β-Ketoacyl-acyl Carrier Protein Synthase III (FabH) with Lauroyl-coenzyme A. J. Mol. Biol. 2005, 346, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Zhou, Y.; Flavin, M.T.; Zhou, L.-M.; Nie, W.; Chen, F.-C. Chalcones and flavonoids as anti-Tuberculosis agents. Bioorg. Med. Chem. 2002, 10, 2795–2802. [Google Scholar] [CrossRef]
- Moodley, S.; Koorbanally, N.A.; Moodley, T.; Ramjugernath, D.; Pillay, M. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay is a rapid, cheap, screening test for the in vitro anti-tuberculous activity of chalcones. J. Microbiol. Methods 2014, 104, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ávila, H.P.; Smânia, E.F.A.; Monache, F.D.; Smânia, A. Structure–activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.M.; Seenivasan, S.P.; Kumar, V.; Doble, M. Synthesis, antimycobacterial activity evaluation, and QSAR studies of chalcone derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Ventura, T.; Calixto, S.; de Azevedo Abrahim-Vieira, B.; de Souza, A.; Mello, M.; Rodrigues, C.; Soter de Mariz e Miranda, L.; Alves de Souza, R.; Leal, I.; Lasunskaia, E.; et al. Antimycobacterial and Anti-Inflammatory Activities of Substituted Chalcones Focusing on an Anti-Tuberculosis Dual Treatment Approach. Molecules 2015, 20, 8072–8093. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Shah, B.H.; Mahmood, A.; Young, D.B.; Hussain, R. The effect of mycobacterial virulence and viability on MAP kinase signalling and TNFα production by human monocytes. Tuberculosis 2003, 83, 299–309. [Google Scholar] [CrossRef]
- O’Kane, C.M.; Elkington, P.T.; Jones, M.D.; Caviedes, L.; Tovar, M.; Gilman, R.H.; Stamp, G.; Friedland, J.S. STAT3, p38 MAPK, and NF-κB Drive Unopposed Monocyte-Dependent Fibroblast MMP-1 Secretion in Tuberculosis. Am. J. Respir. Cell Mol. Biol. 2010, 43, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, D.; Jeong, M.-C.; Jnawali, H.N.; Kwak, C.; Ryoo, S.; Jung, I.D.; Kim, Y. Phloretin Exerts Anti-Tuberculosis Activity and Suppresses Lung Inflammation. Molecules 2017, 22, 183. https://doi.org/10.3390/molecules22010183
Jeon D, Jeong M-C, Jnawali HN, Kwak C, Ryoo S, Jung ID, Kim Y. Phloretin Exerts Anti-Tuberculosis Activity and Suppresses Lung Inflammation. Molecules. 2017; 22(1):183. https://doi.org/10.3390/molecules22010183
Chicago/Turabian StyleJeon, Dasom, Min-Cheol Jeong, Hum Nath Jnawali, Chulhee Kwak, Sungwon Ryoo, In Duk Jung, and Yangmee Kim. 2017. "Phloretin Exerts Anti-Tuberculosis Activity and Suppresses Lung Inflammation" Molecules 22, no. 1: 183. https://doi.org/10.3390/molecules22010183
APA StyleJeon, D., Jeong, M. -C., Jnawali, H. N., Kwak, C., Ryoo, S., Jung, I. D., & Kim, Y. (2017). Phloretin Exerts Anti-Tuberculosis Activity and Suppresses Lung Inflammation. Molecules, 22(1), 183. https://doi.org/10.3390/molecules22010183