Preparative Separation and Purification of Four Glycosides from Gentianae radix by High-Speed Counter-Current Chromatography and Comparison of Their Anti-NO Production Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of HPLC Conditions
2.2. HSCCC Separation
2.3. Identification of the Isolated Compounds
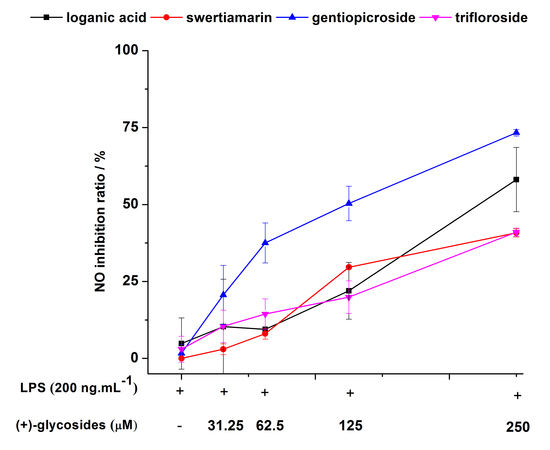
2.4. Inhibition of LPS-Induced NO by Glycosides
2.5. Effects of Glycosides on Cell Viability
3. Experimental
3.1. Apparatus
3.2. Chemicals and Reagents
3.3. Preparation of the Crude Extract
3.4. Selection and Preparation of a Two-Phase Solvent System for HSCCC
3.5. HSCCC Separation Procedure
3.6. HPLC Analysis of the HSCCC Peaks
3.7. Activity of Reducing LPS-Induced NO Production in BV-2 Cells
3.7.1. BV-2 Cell Culture
3.7.2. Nitric Oxide (NO) Assay
3.7.3. Cell Viability Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; Part 1; China Medical Science Press: Beijing, China, 2015; p. 96. [Google Scholar]
- Duan, B.; Hu, J.; Huang, L.; Yang, X.; Chen, F. Chemical fingerprint analysis of Gentianae Radix et Rhizoma by high-performance liquid chromatography. Acta Pharm. Sin. B 2012, 2, 46–52. [Google Scholar] [CrossRef]
- Chang-Liao, W.L.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Isolation of gentiopicroside from Gentianae Radix and its pharmacokinetics on liver ischemia/reperfusion rats. J. Ethnopharmacol. 2012, 141, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.W.; Wong, K.L.; Chan, Y.M.; Xu, H.X.; But, P.P.H.; Shaw, P.C. Isolation of iridoid and secoiridoid glycosides and comparative study on Radix gentianae and related adulterants by HPLC analysis. Phytochemistry 2005, 66, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, W.; Shim, S.H.; Kim, Y.H. Chemical constituents of the rhizomes and roots of Gentiana scabra (Gentianaceae). Biochem. Syst. Ecol. 2015, 61, 169–174. [Google Scholar] [CrossRef]
- Ikeshiro, Y.; Mase, I.; Tomita, Y. A secoiridoid glucoside from Gentiana scabra var. buergeri. Planta Med. 1990, 56, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Jaishree, V.; Badami, S. Antioxidant and hepatoprotective effect of swertiamarin from Enicostemma axillare against d-galactosamine induced acute liver damage in rats. J. Ethnopharmacol. 2010, 130, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Takano, F.; Hojo, H. Suppression of chemically and immunologically induced hepatic injuries by gentiopicroside in mice. Planta Med. 1994, 60, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Bah, M.; Rojas, J.I.; Gutiérrez, D.M. Smooth muscle relaxing activity of gentiopicroside isolated from Gentiana spathacea. Planta Med. 2000, 66, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.C.; Zhang, X.N.; Guo, Y.Y.; Xu, Z.H.; Cao, W.; Sun, X.L.; Sun, W.J.; Zhao, M.G. Down-regulation of NR2B receptors partially contributes to analgesic effects of Gentiopicroside in persistent inflammatory pain. Neuropharmacology 2008, 54, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H.B.; Ahmed, A.A.; Goyal, R.K.; Cheema, S.K. Glycogen phosphorylase-a is a common target for anti-diabetic effect of iridoid and secoiridoid glycosides. J. Pharm. Pharm. Sci. 2013, 16, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Duan, W.; Liu, F.; Shi, X.; Geng, Y.; Wang, X.; Yang, B. Preparative separation of polyphenols from the flowers of Paeonia lactiflora Pall. by high-speed counter-current chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 947, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Geng, Y.; Li, F.; Zheng, C. Isolation and purification of honokiol and magnolol from cortex Magnoliae officinalis by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1036, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ze-Kun, H.; Ni, S.F.; Sun, W.J. Preparative Separation of Gentiopicroside from Aerial Part of Gentiana Macrophylla Pall by High Speed Counter-Current Chromatography. Chin. J. Anal. Chem. 2007, 35, 883–886. [Google Scholar]
- Cheng, H.Y. Separation and Preparation of Gentiopicroside and Sweroside from the Extracts of Swertia franchetiana H. Smith by High Speed Counter-current Chromatography. J. Anhui Agric. Sci. 2010, 1, 38–40. [Google Scholar]
- Liang, J.R.; Ito, Y.; Sun, W.J. Rapid preparative separation of six bioactive compounds from Gentiana crassicaulis Duthie ex Burk. using microwave-assisted extraction coupled with high-speed counter-current chromatography. J. Sep. Sci. 2013, 36, 3934–3940. [Google Scholar] [CrossRef] [PubMed]
- Rho, T.; Jung, M.; Lee, M.W.; Chin, Y.W.; Yoon, K.D. Efficient methods for isolating five phytochemicals from Gentiana macrophylla using high-performance countercurrent chromatography. J. Sep. Sci. 2016, 39, 4723–4731. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Islam, V.H.; Babu, N.P.; Pandikumar, P.; Thirugnanasambantham, K.; Chellappandian, M.; Raj, C.S.D.; Paulraj, M.G.; Ignacimuthu, S. Swertiamarin attenuates inflammation mediators via modulating NF-κB/I κB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur. J. Pharm. Sci. 2014, 56, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.T.; Zhao, Y.P.; Jiao, Y.F.; Zheng, J.; Yang, W.L.; Zhou, R.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Protective effect of gentiopicroside against dextran sodium sulfate induced colitis in mice. Int. Immunopharmacol. 2016, 39, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, Q.; Yu, J.; Jiang, X.; Wu, Z.; Wang, M.; Chen, M.; Chen, X. One-step separation of nine structural analogues from Poria cocos (Schw.) Wolf. via tandem high-speed counter-current chromatography. J. Chromatogr. B 2015, 1004, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xin, X.; Lan, R.; Yuan, Q.; Wang, X.; Li, Y. Isolation of cyanidin 3-glucoside from blue honeysuckle fruits by high-speed counter-current chromatography. Food Chem. 2014, 152, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, Z.; Duan, W.; Fang, L.; Xu, S.; Wang, X. Isolation and purification of seven lignans from Magnolia sprengeri by high-speed counter-current chromatography. J. Chromatogr. B 2011, 879, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Lahloub, M.F.; Sticher, O. Loganin, loganic acid and periclymenoside, a new biosidic ester iridoid glucoside from Lonicera periclymenum L.(Caprifoliaceae). Helv. Chim. Acta 1984, 67, 160–165. [Google Scholar] [CrossRef]
- Rana, V. Separation and Identification of Swertiamarin from Enicostema axillare Lam. Raynal by Centrifugal Partition Chromatography and Nuclear Magnetic Resonance-Mass Spectrometry. J. Pharm. Sci. Emerg. Drugs 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Dijoux-Franca, M.; Mariotte, A.; Amanzadeh, Y.; Sadat-Ebrahimi, S.; Ghazi-Khansari, M.; Mozaffarian, V. Phytochemical study of Swertia longifolia. J. Pharm. Sci. 2015, 16, 245–249. [Google Scholar]
- Kim, J.A.; Son, N.S.; Son, J.K.; Jahng, Y.; Chang, H.W.; Jang, T.S.; Na, M.; Lee, S.H. Two new secoiridoid glycosides from the rhizomes of Gentiana scabra Bunge. Arch. Pharm. Res. 2009, 32, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M.G. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Erpenbeck, V.J.; Jörres, R.A.; Discher, M.; Krentel, H.; Tsikas, D.; Luettig, B.; Krug, N.; Hohlfeld, J.M. Local nitric oxide levels reflect the degree of allergic airway inflammation after segmental allergen challenge in asthmatics. Nitric Oxide 2005, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.; Jiang, W.; Zhang, Y. Isolation and identification of constituents with activity of inhibiting nitric oxide production in RAW 264.7 macrophages from Gentiana triflora. Planta Med. 2013, 79, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, K.J.; Cheng, C.S.; Yan, G.Q.; Lu, W.L.; Ge, J.F.; Cheng, Y.X.; Li, N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014, 153, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ye, J.; Wu, G.T.; Peng, X.J.; Xia, P.F.; Ren, Y. Gentiopicroside prevents interleukin-1 beta induced inflammation response in rat articular chondrocyte. J. Ethnopharmacol. 2015, 172, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, Q.; Wang, M.; Jiang, S.; Zhang, L.; He, X.; Wang, J.; Chen, X. Target-guided separation of antioxidants from Semen cassia via off-line two-dimensional high-speed counter-current chromatography combined with complexation and extrusion elution mode. J. Chromatogr. B 2015, 1001, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Luo, C.; Wang, L.; Wang, J.; Zheng, B.; Peng, Y.; Zhou, L. Preparative separation of 3-O-methylkaempferol from Caragana leucophloea by high-speed counter-current chromatography and its antimicrobial activity. J. Med. Plants Res. 2012, 6, 2081–2087. [Google Scholar]
- Wang, X.; Zhang, Y.; Peng, Y.; Hutchinson, M.; Rice, K.; Yin, H.; Watkins, L. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol. 2016, 173, 856–869. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds loganic acid, swertiamarin, gentiopicroside, and trifloroside are available from the authors. |







| Solvent Systems (v/v) | K Values | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| ethyl acetate–n-butanol–water (4:1:5) | 0.22 | 0.35 | 0.54 | 11.92 |
| ethyl acetate–n-butanol–water (2:1:3) | 0.41 | 0.56 | 0.73 | 9.82 |
| hexane–ethyl acetate–methanol–water (1:1:1:1) | 0.49 | 9.00 | 0.00 | 0.01 |
| hexane–ethyl acetate–methanol–water (1:3:1:3) | 0.50 | 3.54 | 0.01 | 1.89 |
| hexane–ethyl acetate–methanol–water (1:4:1:4) | 0.55 | 2.95 | 0.17 | 3.78 |
| Position | Compounds | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1 | 96.0 | 96.4 | 96.4 | 95.8 |
| 3 | 150.0 | 152.0 | 148.8 | 149.1 |
| 4 | 112.6 | 108.1 | 103.3 | 105.6 |
| 5 | 30.9 | 64.1 | 125.0 | 27.4 |
| 6 | 41.8 | 32.1 | 116.1 | 24.3 |
| 7 | 73.1 | 69.95 | 69.2 | 68.3 |
| 8 | 40.5 | 132.9 | 134.0 | 132.0 |
| 9 | 44.7 | 49.9 | 44.4 | 41.2 |
| 10 | 13.6 | 120.4 | 117.9 | 120.9 |
| 11 | 168.1 | 164.4 | 162.8 | 164.5 |
| 1′ | 98.5 | 98.3 | 98.8 | 96.8 |
| 2′ | 72.1 | 72.9 | 72.8 | 71.3 |
| 3′ | 76.8 | 77.4 | 77.4 | 70.8 |
| 4′ | 70.1 | 70.0 | 70.0 | 70.1 |
| 5′ | 77.2 | 76.1 | 76.6 | 71.7 |
| 6′ | 61.1 | 60.9 | 61.2 | 61.9 |
| 1′′ | 115.6 | |||
| 2′′ | 151.2 | |||
| 3′′ | 146.3 | |||
| 4′′ | 123.3 | |||
| 5′′ | 119.0 | |||
| 6′′ | 121.1 | |||
| 7′′ | 166.1 | |||
| 1′′′ | 102.0 | |||
| 2′′′ | 73.5 | |||
| 3′′′ | 76.3 | |||
| 4′′′ | 69.0 | |||
| 5′′′ | 77.5 | |||
| 6′′′ | 61.0 | |||
| OCOCH3 | 170.3 | |||
| 169.8 | ||||
| 169.1 | ||||
| OCOCH3 | 20.8 | |||
| 20.6 | ||||
| 20.5 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Peng, Y.; Wang, X.; Li, Z.; Sun, Y. Preparative Separation and Purification of Four Glycosides from Gentianae radix by High-Speed Counter-Current Chromatography and Comparison of Their Anti-NO Production Effects. Molecules 2017, 22, 2002. https://doi.org/10.3390/molecules22112002
Chen B, Peng Y, Wang X, Li Z, Sun Y. Preparative Separation and Purification of Four Glycosides from Gentianae radix by High-Speed Counter-Current Chromatography and Comparison of Their Anti-NO Production Effects. Molecules. 2017; 22(11):2002. https://doi.org/10.3390/molecules22112002
Chicago/Turabian StyleChen, Bao, Yinghua Peng, Xinhui Wang, Zhiman Li, and Yinshi Sun. 2017. "Preparative Separation and Purification of Four Glycosides from Gentianae radix by High-Speed Counter-Current Chromatography and Comparison of Their Anti-NO Production Effects" Molecules 22, no. 11: 2002. https://doi.org/10.3390/molecules22112002
APA StyleChen, B., Peng, Y., Wang, X., Li, Z., & Sun, Y. (2017). Preparative Separation and Purification of Four Glycosides from Gentianae radix by High-Speed Counter-Current Chromatography and Comparison of Their Anti-NO Production Effects. Molecules, 22(11), 2002. https://doi.org/10.3390/molecules22112002