New Tripentone Analogs with Antiproliferative Activity
Abstract
:1. Introduction
2. Results and Discussion
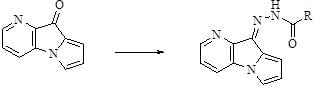
2.1. Chemisty
2.2. Biology
2.2.1. Cytotoxic Activity
2.2.2. Cell Death
2.2.3. Cell Cycle Analysis
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Aminopyridine-Carboxylates (10)
3.1.2. Synthesis of Ethyl 3-(1H-Pyrrol-1-yl)pyridine-2-carboxylate (11)
3.1.3. Synthesis of 2-(Pyrrolidin-1-yl)-3-(1H-pyrrol-1-yl)pyridine (12)
3.1.4. Synthesis of 9H-Pyrido[2,3-b]pyrrolizin-9-one (9a)
3.1.5. Synthesis of Substituted 9H-Pyrido[2,3-b]pyrrolizin-9-ylidenes (9b,d,e,h)
3.1.6. Synthesis of Substituted-9H-pyrido[2,3-b]pyrrolizin-9-ylidenes (9c,f,g)
3.2. Biology
3.2.1. Viability Assay In Vitro
3.2.2. Measurement of Phosphatidylserine (PS) Exposure
3.2.3. Cell Cycle Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antoni, S.; Soerjomataram, I.; Braya, F.; Ferlaya, J.; Møller, B. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull. World Heath Organ. 2016, 94, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Pennati, M.; Parrino, B.; Carbone, A.; Montalbano, A.; Cilibrasi, V.; Zuco, V.; Lopergolo, A.; Cominetti, D.; Diana, P.; et al. Preclinical Activity of New [1,2]Oxazolo[5,4-e]isoindole derivatives in Diffuse Malignant Peritoneal Mesothelioma. J. Med. Chem. 2016, 59, 7223–7238. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G.; Carbone, A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017, 138, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Attanzio, A.; Cascioferro, S.; Carbone, A.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. Synthesis and antitumor activity of new thiazole nortopsentin analogs. Mar. Drugs 2016, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Salvador, A.; Brun, P.; Vedaldi, D.; Diana, P.; Cirrincione, G.; Barraja, P. Pyrazolo[3,4-h]quinolines promising photosensitizing agents in the treatment of cancer. Eur. J. Med. Chem. 2015, 102, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Diana, P.; Stagno, A.; Barraja, P.; Carbone, A.; Parrino, B.; Dall’Acqua, F.; Vedaldi, D.; Salvador, A.; Brun, P.; Castagliuolo, I.; et al. Synthesis of Triazeno-azaindoles a New Class of Triazenes with Antitumor Activity. ChemMedChem 2011, 6, 1291–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spanò, V.; Sbarra, S.; Doldi, V.; De Cesare, M.; et al. Novel 1H-Pyrrolo[2,3-b]pyridine Derivative Nortopsentin Analogues: Synthesis and Antitumor Activity in Peritoneal Mesothelioma Experimental Models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Giallombardo, D.; Cilibrasi, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Frasson, I.; Salvador, A.; Richter, S.N.; Doria, F.; et al. Pyrrolo[3′,2′:6,7]cyclohepta[1,2-b]pyridines with potent photo-antiproliferative activity. Eur. J. Med. Chem. 2017, 128, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Frasson, I.; Giallombardo, D.; Doria, F.; Parrino, B.; Carbone, A.; Montalbano, A.; Nadai, M.; Diana, P.; Cirrincione, G.; et al. Synthesis and antiproliferative mechanism of action of pyrrolo[3′,2′:6,7]cyclohepta[1,2-d]pyrimidin-2-amines as singlet oxygen photosensitizers. Eur. J. Med. Chem. 2016, 123, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Di Vita, G.; Ciancimino, C.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; et al. 3-[4-(1H-Indol-3-yl)-1,3-thiazol-2-yl]-1H-pyrrolo[2,3-b]pyridines, Nortopsentin Analogues with Antiproliferative Activity. Mar. Drugs 2015, 13, 1901–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, A.; Parrino, B.; Di Vita, G.; Attanzio, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Livrea, M.A.; Diana, P.; et al. Synthesis and antiproliferative activity of thiazolyl-bis-pyrrolo[2,3-b]pyridines and indolyl-thiazolyl-pyrrolo[2,3-c]pyridines, nortopsentin analogues. Mar. Drugs 2015, 13, 460–492. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Spanò, V.; Carbone, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Montalbano, A. Synthesis of the New Ring System Bispyrido[4′,3′:4,5]pyrrolo[1,2-a:1′,2′-d]pyrazine and Its Deaza Analogue. Molecules 2014, 19, 13342–13347. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Diana, P.; Cirrincione, G.; Castagliuolo, I.; Brun, P.; Issinger, O.G.; Tisi, S.; et al. Synthesis of a new class of pyrrolo[3,4-h]quinazolines with antimitotic activity. Eur. J. Med. Chem. 2014, 74, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Barraja, P.; Montalbano, A.; Parrino, B.; Spanò, V.; Lopergolo, A.; Sbarra, S.; Doldi, V.; Zaffaroni, N.; et al. Synthesis and Antiproliferative Activity of Substituted 3[2-(1H-indol-3-yl)-1,3-Thiazol-4-yl]-1H-Pyrrolo[3,2-b]Pyridines, Marine Alkaloid Nortopsentin Analogues. Curr. Med. Chem. 2014, 21, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Diana, P.; Cirrincione, G.; Barraja, P. Convenient synthesis of pyrrolo[3,4-g]indazole. Tetrahedron 2013, 69, 9839–9847. [Google Scholar] [CrossRef]
- Montalbano, A.; Parrino, B.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Cirrincione, G. Synthesis of the new oligopeptide pyrrole derivative isonetropsin and its one pyrrole unit analogue. Tetrahedron 2013, 69, 2550–2554. [Google Scholar] [CrossRef]
- Barraja, P.; Diana, P.; Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. An efficient synthesis of pyrrolo[3′,2′:4,5]thiopyrano[3,2-b]pyridin-2-one: A new ring system of pharmaceutical interest. Tetrahedron 2012, 68, 5087–5094. [Google Scholar] [CrossRef]
- Carbone, A.; Parrino, B.; Barraja, P.; Spanò, V.; Cirrincione, G.; Diana, P.; Maier, A.; Kelter, G.; Fiebig, H.-H. Synthesis and antiproliferative activity of 2,5-bis(3′-indolyl)pyrroles, analogues of the marine alkaloid Nortopsentin. Mar. Drugs 2013, 11, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Muscarella, M.; Spanò, V.; Montalbano, A.; Barraja, P.; Salvador, A.; Vedaldi, D.; Cirrincione, G.; Diana, P. 11H-pyrido[3′,2′:4,5]pyrrolo[3,2-c]cinnoline and Pyrido[3′,2′:4,5]pyrrolo[1,2-c][1,2,3]benzotriazine: Two new ring systems with antitumor activity. J. Med. Chem. 2014, 57, 9495–9511. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, J.C.; Letois, B.; Rault, S.; Robba, M.; Rogosca, M. Thienopyrrolizines: New condensed triheterocyclic systems. J. Heterocycl. Chem. 1994, 31, 501–504. [Google Scholar] [CrossRef]
- Lisowski, V.; Leonce, S.; Kraus-Berthier, L.; Sopkova-de Oliveira, S.J.; Pierre, A.; Atassi, G.; Caignard, D.H.; Renard, P.; Rault, S. Design, synthesis, and evaluation of novel thienopyrrolizinones as antitubulin agents. J. Med. Chem. 2004, 47, 1448–1464. [Google Scholar] [CrossRef] [PubMed]
- Rochais, C.; Duc, N.V.; Lescot, E.; Sopkova-de Oliveira, S.J.; Bureau, R.; Meijer, L.; Dallemagne, P.; Rault, S. Synthesis of new dipyrrolo- and furopyrrolopyrazinones related to tripentone and their biological evaluation as potential kinases (CDKs1–5, GSK3) inhibitors. Eur. J. Med. Chem. 2009, 44, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Rochais, C.; Lisowski, V.; Dallemagne, P.; Rault, S. Synthesis and biological evaluation of novel pyrrolopyrolizinones as anticancer agents. Bioorg. Med. Chem. 2006, 14, 8162–8175. [Google Scholar] [CrossRef] [PubMed]
- Rochais, C.; Lisowski, V.; Dallemagne, P.; Rault, S. First synthesis of arylpyrrolo- and pyrazolopyrrolizinones as useful agents with potential biological interest. Tetrahedron Lett. 2004, 45, 6353–6355. [Google Scholar] [CrossRef]
- Diana, P.; Stagno, A.; Barraja, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of the new ring system pyrrolizino[2,3-b]indol-4(5H)-one. Tetrahedron 2011, 67, 3374–3379. [Google Scholar] [CrossRef]
- Rochais, C.; Cresteil, T.; Perri, V.; Jouanne, M.; Lesnard, A.; Rault, S.; Dallemagne, P. MR22388, a novel anti-cancer agent with a strong FLT-3 ITD kinase affinity. Cancer Lett. 2013, 331, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Spanò, V.; Montalbano, A.; Giallombardo, D.; Barraja, P.; Attanzio, A.; Tesoriere, L.; Sissi, C.; Palumbo, M.; et al. Aza-isoindolo and isoindolo-azaquinoxaline derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 94, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Ciancimino, C.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P.; Sissi, C.; Palumbo, M.; et al. Water-soluble isoindolo[2,1-a]quinoxalin-6-imines: In vitro antiproliferative activity and molecular mechanism(s) of action. Eur. J. Med. Chem. 2015, 94, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Ciancimino, C.; Carbone, A.; Spanò, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P. Synthesis of isoindolo[1,4]benzoxazinone and isoindolo[1,5]benzoxazepine: Two new ring systems of pharmaceutical interest. Tetrahedron 2015, 71, 7332–7338. [Google Scholar] [CrossRef]
- Spanò, V.; Pennati, M.; Parrino, B.; Carbone, A.; Montalbano, A.; Lopergolo, A.; Zuco, V.; Cominetti, D.; Diana, P.; Cirrincione, G.; et al. [1,2]Oxazolo[5,4-e]isoindoles as promising tubulin polymerization inhibitors. Eur. J. Med. Chem. 2016, 124, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Barraja, P.; Spanò, V.; Giallombardo, G.; Diana, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of [1,2]oxazolo[5,4-e]indazoles as antitumor agents. Tetrahedron 2013, 69, 6474–6477. [Google Scholar] [CrossRef]
- Barraja, P.; Caracausi, L.; Diana, P.; Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis and Antiproliferative Activity of the Ring System [1,2]Oxazolo[4,5-g]indole. ChemMedChem 2012, 7, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Patella, C.; Diana, P.; Barraja, P.; Montalbano, A.; Cirrincione, G.; Dattolo, G.; Almerico, A.M. A new tetracyclic ring system of biological interest. Indolo[3,2-e][1,2,3]triazolo[1,5-a]pyrimidines through domino reactions of 2-azidoindole. Heterocycles 2003, 60, 2269–2275. [Google Scholar]
- Lauria, A.; Diana, P.; Barraja, P.; Almerico, A.M.; Cirrincione, G.; Dattolo, G. Pyrrolo[3,4-e][1,2,3]triazolo[1,5-a]pyrimidine and pyrrolo[3,4-d][1,2,3]triazolo[1,5-a]pyrimidine. New tricyclic ring systems of biological interest. J. Heterocycl. Chem. 2000, 37, 747–750. [Google Scholar] [CrossRef]
- Grande, F.; Yamada, R.; Cao, X.; Aiello, F.; Garofalo, A.; Neamati, N. Synthesis and biological evaluation of novel hydrazide based cytotoxic agents. Expert Opin. Investig. Drugs 2009, 18, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Carpino, L.A.; Xia, J.; El-Faham, A. 3-Hydroxy-4-oxo-3, 4-dihydro-5-azabenzo-1,2,3-triazene. J. Org. Chem. 2004, 69, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lennernas, H.; Welage, L.S.; Barnett, J.L.; Landowski, C.P.; Foster, D.; Fleischer, D.; Lee, K.D.; Amidon, G.L. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequence tags and correlation with permeability of 26 drugs. Pharm. Res. 2002, 19, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
- Girasolo, M.A.; Attanzio, A.; Sabatino, P.; Tesoriere, L.; Rubino, S.; Stocco, G. Organotin(IV) derivatives with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidine and their cytotoxic activities: The importance of being conformers. Inorg. Chim. Acta 2014, 423, 168–176. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds 9a–h are available from the authors. |





| Compound | R | Yield (%) |
|---|---|---|
| 9a | - | 70 |
| 9b | Pyridin-4-yl | 69 |
| 9c | Pyridin-3-yl | 93 |
| 9d | Furan-2-yl | 72 |
| 9e | Thiophen-2-yl | 70 |
| 9f | 4-NH2-phenyl | 60 |
| 9g | 4-OH-phenyl | 90 |
| 9h | Phenyl | 76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrino, B.; Ullo, S.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Cirrincione, G.; Diana, P. New Tripentone Analogs with Antiproliferative Activity. Molecules 2017, 22, 2005. https://doi.org/10.3390/molecules22112005
Parrino B, Ullo S, Attanzio A, Spanò V, Cascioferro S, Montalbano A, Barraja P, Tesoriere L, Cirrincione G, Diana P. New Tripentone Analogs with Antiproliferative Activity. Molecules. 2017; 22(11):2005. https://doi.org/10.3390/molecules22112005
Chicago/Turabian StyleParrino, Barbara, Salviana Ullo, Alessandro Attanzio, Virginia Spanò, Stella Cascioferro, Alessandra Montalbano, Paola Barraja, Luisa Tesoriere, Girolamo Cirrincione, and Patrizia Diana. 2017. "New Tripentone Analogs with Antiproliferative Activity" Molecules 22, no. 11: 2005. https://doi.org/10.3390/molecules22112005
APA StyleParrino, B., Ullo, S., Attanzio, A., Spanò, V., Cascioferro, S., Montalbano, A., Barraja, P., Tesoriere, L., Cirrincione, G., & Diana, P. (2017). New Tripentone Analogs with Antiproliferative Activity. Molecules, 22(11), 2005. https://doi.org/10.3390/molecules22112005