Multi-Component One-Pot Reaction of Aromatic Carbonyl Compounds, Tosylhydrazide, and Arylboronic Acids
Abstract
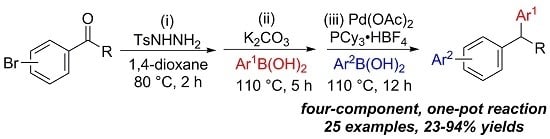
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for the One-Pot, Four-Component Reactions of 4-Bromoacetophenone, TsNHNH2, and Two Same Arylboronic Acids
3.2. General Procedure for the One-Pot, Four-Step, Four-Component Reactions of 4-Acetylbiphenylderivatives, TsNHNH2, and Two Different Arylboronic Acids
3.3. General Procedure for the Synthesis of 3x and 3y
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanabe, K.; Hölderich, W.F. Industrial application of solid acid-base catalysts. Appl. Catal. A 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Beltrame, P.; Zuretti, G.; Demartin, F. Benzylation of biphenyl with benzyl chloride over crystalline, amorphous, and MCM-41 solid acid catalysts. Ind. Eng. Chem. Res. 2000, 39, 1209–1214. [Google Scholar] [CrossRef]
- Hino, M.; Arata, K. The synthesis of thermally stable oils by the benzylation of biphenyl with benzyl chloride catalyzed by Iron(III) oxide. Bull. Chem. Soc. Jpn. 1981, 54, 311–312. [Google Scholar] [CrossRef]
- Huhtaniemi, I.; Nikula, H.; Parvinen, M.; Rannikko, S. Histological and functional changes of the testis tissue during GnRH agonist treatment of prostatic cancer. Am. J. Clin. Oncol. 1988, 11, S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Titus, M.A.; Schell, M.J.; Lih, F.B.; Tomer, K.B.; Mohler, J.L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 2005, 11, 4653–4657. [Google Scholar] [CrossRef] [PubMed]
- Stanbrough, M.; Bubley, G.J.; Ross, K.; Golub, T.R.; Rubin, M.A.; Penning, T.M.; Febbo, P.G.; Balk, S.P. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006, 66, 2815–2825. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Holzbeierlein, J.; Lal, P.; La Tulippe, E.; Smith, A.; Satagopan, J.; Zhang, L.; Ryan, C.; Smith, S.; Scher, H.; Scardino, P.; et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am. J. Pathol. 2004, 164, 217–227. [Google Scholar] [CrossRef]
- Labrie, F.; Dupont, A.; Belanger, A.; Cusan, L.; Lacourciere, Y.; Monfette, G.; Laberge, J.G.; Emond, J.P.; Fazekas, A.T.; Raynaud, J.P.; et al. New hormonal therapy in prostatic carcinoma: Combined treatment with an LHRH agonist and an antiandrogen. Clin. Investig. Med. 1982, 5, 267–275. [Google Scholar]
- Hu, Q.Z.; Yin, L.; Jagusch, C.; Hille, U.E.; Hartmann, R.W. Isopropylidene substitution increases activity and selectivity of biphenylmethylene 4-pyridine type CYP17 inhibitors. J. Med. Chem. 2010, 53, 5049–5053. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.Z.; Jagusch, C.; Hille, U.E.; Haupenthal, J.; Hartmann, R.W. Replacement of imidazolyl by pyridyl in biphenylmethylenes results in selective CYP17 and dual CYP17/CYP11B1 inhibitors for the treatment of prostate cancer. J. Med. Chem. 2010, 53, 5749–5758. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.D.; George, G. Monoalkylation of biphenyl over modified heteropoly acids: Novelty of cesium substituted dodecatungstophosphoric acid supported on hexagonal mesoporous silica. Catal. Today 2009, 141, 130–137. [Google Scholar] [CrossRef]
- Beltrame, P.; Demartin, F.; Zuretti, G. An improved kinetic model for the reaction of biphenyl with benzyl chloride over an MCM-41 solid acid catalyst. Appl. Catal. A Gen. 2001, 218, 61–67. [Google Scholar] [CrossRef]
- Jana, S.K. Recent developments of heterogeneous solid catalysts for liquid-phase Friedel-Crafts type benzylation reaction. Catal. Surv. Asia 2005, 9, 25–34. [Google Scholar] [CrossRef]
- Haase, J.; Hillner, K.; Brueggemann, W.; Momm, G. p-Benzylbiphenyl. Eur. Pat. Appl. Eur. Patent 431,265, 12 June 1991. [Google Scholar]
- Sakura, K.; Takeuchi, H.; Furumoto, M. Manufacture of Benzylbiphenyls. Jpn. Kokai Tokkyo Koho JP 03,170,442, 24 July 1991. [Google Scholar]
- Beltrame, P.; Zuretti, G. Benzylation of biphenyl with benzyl chloride over HY zeolites: A kinetic model for reaction and catalyst deactivation. Ind. Eng. Chem. Res. 1997, 36, 3427–3432. [Google Scholar] [CrossRef]
- Miyaura, N.; Yanagi, T.; Suzuki, A. The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases. Synth. Commun. 1981, 11, 513–519. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions via organoboranes. J. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Langle, S.; Abarbri, M.; Duchêne, A. Selective double Suzuki cross-coupling reactions. Synthesis of unsymmetrical diaryl (or heteroaryl) methanes. Tetrahedron Lett. 2003, 44, 9255–9258. [Google Scholar] [CrossRef]
- Henry, N.; Enguehard-Gueiffier, C.; Thery, I.; Gueiffier, A. One-pot dual substitutions of bromobenzyl chloride, 2-chloromethyl-6-halogenoimidazo [1,2-a] pyridine and-[1,2-b]pyridazine by Suzuki-Miyaura cross-coupling reactions. Eur. J. Org. Chem. 2008, 2008, 4824–4827. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaym, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.L. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Yang, T.; Cui, H.; Zhang, C.H.; Zhang, L.; Su, C.Y. From homogeneous to heterogeneous catalysis of the three-component coupling of oxysulfonyl azides, alkynes, and amines. ChemCatChem 2013, 5, 3131–3138. [Google Scholar] [CrossRef]
- Fulton, J.R.; Aggarwal, V.K.; de Vicente, J. The use of tosylhydrazone salts as a safe alternative for handling diazo compounds and their applications in organic synthesis. Eur. J. Org. Chem. 2005, 1479–1492. [Google Scholar] [CrossRef]
- Barluenga, J.; Valdés, C. Tosylhydrazones: New uses for classic reagents in palladium-catalyzed cross-coupling and metal-free reactions. Angew. Chem. Int. Ed. 2011, 50, 7486–7500. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhang, H. N-tosylhydrazones: Versatile reagents for metal-catalyzed and metal-free cross-coupling reactions. Chem. Soc. Rev. 2012, 41, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Kai, X.; Chong, S.; Shang, S. Progress of cross-coupling reaction with N-tosylhydrazones. Chin. J. Org. Chem. 2015, 35, 294–308. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Wang, J. Transition-metal-catalyzed cross-coupling reaction with N-tosylhydrazones. Chin. J. Org. Chem. 2013, 33, 687–692. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, Y.; Wang, J. Diazo compounds and N-tosylhydrazones: Novel cross-coupling partners in transition-metal-catalyzed reactions. Acc. Chem. Res. 2013, 46, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.P.; Ray, D.; BhaskaraRao, V.U.; Singh, R.P. Copper-catalyzed direct cross-coupling of compounds containing activated C–H/heteroatom–H bonds with N-tosylhydrazones. Eur. J. Org. Chem. 2016, 2369–2382. [Google Scholar] [CrossRef]
- Barluenga, J.; Tomás-Gamasa, M.; Aznar, F.; Valdés, C. Metal-free carbon–carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. Nat. Chem. 2009, 1, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Shi, Y.; Xiao, Q.; Liu, Y.; Ye, F.; Zhang, Y.; Wang, J. CuBr-Catalyzed coupling of N-tosylhydrazones and terminal alkynes: Synthesis of benzofurans and indoles. Org. Lett. 2011, 13, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Shi, Y.; Zhou, L.; Xiao, Q.; Zhang, Y.; Wang, J. Expeditious synthesis of phenanthrenes via CuBr2-catalyzed coupling of terminal alkynes and N-tosylhydrazones derived from O-formyl biphenyls. Org. Lett. 2011, 13, 5020–5023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, T.; Dong, X. Benzofuran and indole synthesis via Cu(I)-catalyzed coupling of N-tosylhydrazone and o-hydroxy or o-amino phenylacetylene. Org. Biomol. Chem. 2013, 11, 1490–1497. [Google Scholar]
- Zhou, L.; Ye, F.; Zhang, Y.; Wang, J. Pd-catalyzed three-component coupling of N-tosylhydrazone, terminal alkyne, and aryl halide. J. Am. Chem. Soc. 2010, 132, 13590–13591. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Hamze, A.; Brion, J.-D.; Alami, M. Catalytic three-component one-pot reaction of hydrazones, dihaloarenes, and amines. Org. Lett. 2013, 15, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Naret, T.; Retailleau, P.; Bignon, J.; Brion, J.-D.; Alami, M.; Hamze, A. Palladium-catalyzed one-pot synthesis of 5-(1-arylvinyl)-1H-benzimidazoles: Overcoming the limitation of acetamide partners. Adv. Synth. Catal. 2016, 358, 1833–1847. [Google Scholar] [CrossRef]
- Shen, X.; Gu, N.; Liu, P.; Ma, X.; Xie, J.; Liu, Y.; He, L.; Dai, B. A simple and efficient synthesis of 9-arylfluorenes via metal-free reductive coupling of arylboronic acids and N-tosylhydrazones in situ. RSC Adv. 2015, 5, 63726–63731. [Google Scholar] [CrossRef]
- Shen, X.; Gu, N.; Liu, P.; Ma, X.; Xie, J.; Liu, Y.; Dai, B. One-pot synthesis of triarylmethanes via metal-free reductive coupling of diaryl ketones, tosylhydrazide, and arylboronic acids. Chin. J. Chem. 2016, 34, 1033–1038. [Google Scholar] [CrossRef]
- Shen, X.; Liu, P.; Liu, Y.; Liu, Y.; Dai, B. One-pot reductive coupling reactions of acetyl naphthalene derivatives, tosylhydrazide, with arylboronic acids. Tetrahedron 2017, 73, 785–793. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Liu, Y.; Wei, Y. Pd(0)-catalyzed tandem one-pot reaction of biphenyl ketones/aldehydes to the corresponding di-substituted aryl olefins. Chin. J. Chem. 2017, 35, 1141–1148. [Google Scholar] [CrossRef]
- López-Pérez, A.; Adrio, J.; Carretero, J.C. Palladium-catalyzed cross-coupling reaction of secondary benzylic bromides with Grignard reagents. Org. Lett. 2009, 11, 5514–5517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, M.T.; Lu, J.M. N-Heterocyclic carbine-palladium (ii)-1-methylimidazole complex catalyzed Suzuki-Miyaura coupling of benzylic chlorides with arylboronic acids or potassium phenyltrifluoroborate in neat water. Org. Biomol. Chem. 2013, 11, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, S.E.; Abarbri, M.; Duchêne, A.; Langle-Lamande, S.; Thibonnet, J. Efficient synthesis of substituted styrenes and biaryls (or heteroaryls) with regioselective reactions of ortho-, meta-, and para-bromobenzyl bromide. Synthesis 2012, 44, 2023–2040. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |








| Entry | Pd(OAc)2 | Ligand | PhB(OH)2 | Base | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 1 mol % | - | 2.5 | K2CO3 | 15 |
| 2 | 1 mol % | PPh3 | 2.5 | K2CO3 | 61 |
| 3 | 1 mol % | PCy3∙HBF4 | 2.5 | K2CO3 | 78 |
| 4 | 1 mol % | PCy3∙HBF4 | 2.5 | Et3N | 63 |
| 5 | 1 mol % | PCy3∙HBF4 | 2.5 | K3PO4∙H2O | 71 |
| 6 | 1 mol % | PCy3∙HBF4 | 2.5 | NaOH | 72 |
| 7 | 1 mol % | PCy3∙HBF4 | 2.5 | Cs2CO3 | 28 |
| 8 c | 1 mol % | PCy3∙HBF4 | 3.0 | K2CO3 | 89 |
| 9 | 2 mol % | PCy3∙HBF4 | 3.0 | K2CO3 | 90 |
| 10 d | 1 mol % | PCy3∙HBF4 | 3.0 | K2CO3 | 52 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, N.; Wei, Y.; Liu, P.; Liu, Y.; Dai, B. Multi-Component One-Pot Reaction of Aromatic Carbonyl Compounds, Tosylhydrazide, and Arylboronic Acids. Molecules 2017, 22, 2168. https://doi.org/10.3390/molecules22122168
Gu N, Wei Y, Liu P, Liu Y, Dai B. Multi-Component One-Pot Reaction of Aromatic Carbonyl Compounds, Tosylhydrazide, and Arylboronic Acids. Molecules. 2017; 22(12):2168. https://doi.org/10.3390/molecules22122168
Chicago/Turabian StyleGu, Ningning, Yu Wei, Ping Liu, Yan Liu, and Bin Dai. 2017. "Multi-Component One-Pot Reaction of Aromatic Carbonyl Compounds, Tosylhydrazide, and Arylboronic Acids" Molecules 22, no. 12: 2168. https://doi.org/10.3390/molecules22122168
APA StyleGu, N., Wei, Y., Liu, P., Liu, Y., & Dai, B. (2017). Multi-Component One-Pot Reaction of Aromatic Carbonyl Compounds, Tosylhydrazide, and Arylboronic Acids. Molecules, 22(12), 2168. https://doi.org/10.3390/molecules22122168