Pathogenic Acanthamoeba castellanii Secretes the Extracellular Aminopeptidase M20/M25/M40 Family Protein to Target Cells for Phagocytosis by Disruption
Abstract
:1. Introduction
2. Results
2.1. Acanthamoeba castellanii Induces Cell Damage in C6 Cells
2.2. Cytopathic Effect Process of Acanthamoeba castellanii
2.3. Acanthamoeba Secreted Proteins Involves the Co-Cultured Cell Damage Process
2.4. The Biochemical Characterization of Acanthamoeba Secreted Proteins
2.5. The Discovery of M20/M25/M40 Aminopeptidase by Comparative Analysis of RNA-seq
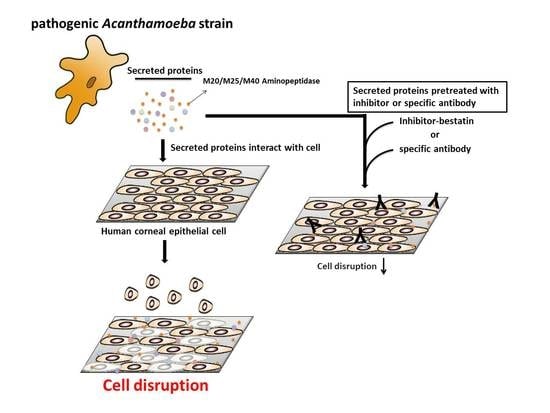
2.6. Aminopeptidase Inhibitor and Antibodies Reduce the Cell Damage
3. Discussion
4. Materials and Methods
4.1. Culture of Acanthamoeba Protozoa
4.2. Assay of Cytopathic Effects (CPE)
4.3. Live Cell Imaging of C6 Cells Co-Incubation with Acanthamoeba
4.4. Isolation of Secreted Proteins
4.5. Rat Glial C6 Cell Treated with Acanthamoeba and Asp
4.6. Biochemical Properties of Asp
4.7. Total RNA Isolation
4.8. cDNA Synthesis
4.9. Reverse Transcription PCR
4.10. Protein Analysis
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Illingworth, C.D.; Cook, S.D. Acanthamoeba keratitis. Surv. Ophthalmol. 1998, 42, 493–508. [Google Scholar] [CrossRef]
- Martinez, A.J.; Visvesvara, G.S. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997, 7, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Shih, M.H.; Huang, F.C.; Tseng, S.H.; Chen, C.C. Treatment of early Acanthamoeba keratitis with alcohol-assisted epithelial debridement. Cornea 2012, 31, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.W.; Niederkorn, J.Y. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006, 22, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef] [PubMed]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Wynter-Allison, Z.; Lorenzo Morales, J.; Calder, D.; Radlein, K.; Ortega-Rivas, A.; Lindo, J.F. Acanthamoeba infection as a cause of severe keratitis in a soft contact lens wearer in Jamaica. Am. J. Trop. Med. Hyg. 2005, 73, 92–94. [Google Scholar] [PubMed]
- Lourenssen, S.; Houpt, E.R.; Chadee, K.; Blennerhassett, M.G. Entamoeba histolytica infection and secreted proteins proteolytically damage enteric neurons. Infect. Immun. 2010, 78, 5332–5340. [Google Scholar] [CrossRef] [PubMed]
- Ocádiz, R.; Orozco, E.; Carrillo, E.; Quintas, L.I.; Ortega-López, J.; García-Pérez, R.M.; Sánchez, T.; Castillo-Juárez, B.A.; García-Rivera, G.; Rodríguez, M.A. EhCP112 is an Entamoeba histolytica secreted cysteine protease that may be involved in the parasite-virulence. Cell. Microbiol. 2005, 7, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Lidell, M.E.; Moncada, D.M.; Chadee, K.; Hansson, G.C. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc. Natl. Acad. Sci. USA 2006, 103, 9298–9303. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Ortega-Rivas, A.; Foronda, P.; Abreu-Acosta, N.; Ballart, D.; Martinez, E.; Valladares, B. RNA interference (RNAi) for the silencing of extracellular serine proteases genes in Acanthamoeba: Molecular analysis and effect on pathogenecity. Mol. Biochem. Parasitol. 2005, 144, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Mattana, A.; Cappai, V.; Alberti, L.; Serra, C.; Fiori, P.L.; Cappuccinelli, P. ADP and other metabolites released from Acanthamoeba castellanii lead to human monocytic cell death through apoptosis and stimulate the secretion of proinflammatory cytokines. Infect. Immun. 2002, 70, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Hurtle, W.; Bode, E.; Kaplan, R.S.; Garrison, J.; Kearney, B.; Shoemaker, D.; Henchal, E.; Norwood, D. Use of denaturing high-performance liquid chromatography to identify Bacillus anthracis by analysis of the 16S-23S rRNA interspacer region and gyrA gene. J. Clin. Microbiol. 2003, 41, 4758–4766. [Google Scholar] [CrossRef] [PubMed]
- Hurt, M.; Niederkorn, J.; Alizadeh, H. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3424–3431. [Google Scholar] [CrossRef]
- Huang, J.M.; Lin, W.C.; Li, S.C.; Shih, M.H.; Chan, W.C.; Shin, J.W.; Huang, F.C. Comparative proteomic analysis of extracellular secreted proteins expressed by two pathogenic Acanthamoeba castellanii clinical isolates and a non-pathogenic ATCC strain. Exp. Parasitol. 2016, 166, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-C.; Shih, M.-H.; Chang, K.-F.; Huang, J.-M.; Shin, J.-W.; Lin, W.-C. Characterizing clinical isolates of Acanthamoeba castellanii with high resistance to polyhexamethylene biguanide in Taiwan. J. Microbiol. Immunol. Infect. 2015, 50, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Lagmay, J.P.; Matias, R.R.; Natividad, F.F.; Enriquez, G.L. Cytopathogenicity of Acanthamoeba isolates on rat glial C6 cell line. Southeast Asian J. Trop. Med. Public Health 1999, 30, 670–677. [Google Scholar] [PubMed]
- Zaidel-Bar, R.; Cohen, M.; Addadi, L.; Geiger, B. Hierarchical Assembly of Cell–Matrix Adhesion Complexes; Portland Press Limited: London, UK, 2004. [Google Scholar]
- Heickendorff, L. Laminin, fibronectin and type IV collagen in BM-like material from cultured arterial smooth muscle cells. Int. J. Biochem. 1988, 20, 381–386. [Google Scholar] [CrossRef]
- Sugrue, S.P.; Hay, E.D. The identification of extracellular matrix (ECM) binding sites on the basal surface of embryonic corneal epithelium and the effect of ECM binding on epithelial collagen production. J. Cell Biol. 1986, 102, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kanzaki, H.; Imai, K.; Narukawa, S.; Higuchi, T.; Katsuragawa, H.; Maeda, M.; Mori, T. Bestatin, a potent aminopeptidase-N inhibitor, inhibits in vitro decidualization of human endometrial stromal cells. J. Clin. Endocrinol. Metab. 1994, 79, 171–175. [Google Scholar] [PubMed]
- Lee, Y.R.; Na, B.K.; Moon, E.K.; Song, S.M.; Joo, S.Y.; Kong, H.H.; Goo, Y.K.; Chung, D.I.; Hong, Y. Essential Role for an M17 Leucine Aminopeptidase in Encystation of Acanthamoeba castellanii. PLoS ONE 2015, 10, e0129884. [Google Scholar] [CrossRef] [PubMed]
- Gavin, H.E.; Beubier, N.T.; Satchell, K.J. The Effector Domain Region of the Vibrio vulnificus MARTX Toxin Confers Biphasic Epithelial Barrier Disruption and Is Essential for Systemic Spread from the Intestine. PLoS Pathog. 2017, 13, e1006119. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y.; Alizadeh, H.; Leher, H.; McCulley, J.P. The pathogenesis of Acanthamoeba keratitis. Microbes Infect. 1999, 1, 437–443. [Google Scholar] [CrossRef]
- Garate, M.; Cao, Z.; Bateman, E.; Panjwani, N. Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J. Biol. Chem. 2004, 279, 29849–29856. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Bates, E.J. Elastase in the pathogenic free-living amoebae Naegleria and Acanthamoeba spp. Infect. Immun. 1988, 56, 3320–3321. [Google Scholar] [PubMed]
- Mortazavi, P.N.; Keisary, E.; Loh, L.N.; Jung, S.-Y.; Khan, N.A. Possible roles of phospholipase A 2 in the biological activities of Acanthamoeba castellanii (T4 genotype). Protist 2011, 162, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Panjwani, N. Pathogenesis of Acanthamoeba keratitis. Ocul. Surf. 2010, 8, 70–79. [Google Scholar] [CrossRef]
- Munoz, M.D.L.; Calderon, J.; Rojkind, M. The collagenase of Entamoeba histolytica. J. Exp. Med. 1982, 155, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Gordon, V.R.; Asem, E.K.; Vodkin, M.H.; McLaughlin, G.L. Acanthamoeba binds to extracellular matrix proteins in vitro. Investig. Ophthalmol. Vis. Sci. 1993, 34, 658–662. [Google Scholar]
- Mitra, M.M.; Alizadeh, H.; Gerard, R.D.; Niederkorn, J.Y. Characterization of a plasminogen activator produced by Acanthamoeba castellanii. Mol. Biochem. Parasitol. 1995, 73, 157–164. [Google Scholar] [CrossRef]
- Martinez-Palomo, A.; Gonzalez-Robles, A.; Chavez, B.; Orozco, E.; Fernandez-Castelo, S.; Cervantes, A. Structural bases of the cytolytic mechanisms of Entamoeba histolytica. J. Protozool. 1985, 32, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Leroy, A.; Lauwaet, T.; De Bruyne, G.; Cornelissen, M.; Mareel, M. Entamoeba histolytica disturbs the tight junction complex in human enteric T84 cell layers. FASEB J. 2000, 14, 1139–1146. [Google Scholar] [PubMed]
- Leroy, A.; Lauwaet, T.; Oliveira, M.-J.; De Bruyne, G.; Bracha, R.; Ankri, S.; Katz, U.; Mirelman, D.; Mareel, M. Disturbance of tight junctions by Entamoeba histolytica: Resistant vertebrate cell types and incompetent trophozoites. Arch. Med. Res. 2000, 31, S218–S220. [Google Scholar] [CrossRef]
- Huang, F.C.; Liu, T.S.; Li, S.C.; Shih, M.H.; Shin, J.W.; Lin, W.C. The effect of the disulfideisomerase domain containing protein in the defense against polyhexamethylene biguanide of highly tolerant Acanthamoeba at the trophozoite stage. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Qvarnstrom, Y.; Visvesvara, G.S.; Sriram, R.; da Silva, A.J. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J. Clin. Microbiol. 2006, 44, 3589–3595. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds the NCBI BioSample (accession number of NCKH_D is SAMN08174149) are available from the authors. |








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-M.; Liao, C.-C.; Kuo, C.-C.; Chen, L.-R.; Huang, L.L.H.; Shin, J.-W.; Lin, W.-C. Pathogenic Acanthamoeba castellanii Secretes the Extracellular Aminopeptidase M20/M25/M40 Family Protein to Target Cells for Phagocytosis by Disruption. Molecules 2017, 22, 2263. https://doi.org/10.3390/molecules22122263
Huang J-M, Liao C-C, Kuo C-C, Chen L-R, Huang LLH, Shin J-W, Lin W-C. Pathogenic Acanthamoeba castellanii Secretes the Extracellular Aminopeptidase M20/M25/M40 Family Protein to Target Cells for Phagocytosis by Disruption. Molecules. 2017; 22(12):2263. https://doi.org/10.3390/molecules22122263
Chicago/Turabian StyleHuang, Jian-Ming, Chen-Chieh Liao, Chung-Ching Kuo, Lih-Ren Chen, Lynn L. H. Huang, Jyh-Wei Shin, and Wei-Chen Lin. 2017. "Pathogenic Acanthamoeba castellanii Secretes the Extracellular Aminopeptidase M20/M25/M40 Family Protein to Target Cells for Phagocytosis by Disruption" Molecules 22, no. 12: 2263. https://doi.org/10.3390/molecules22122263
APA StyleHuang, J. -M., Liao, C. -C., Kuo, C. -C., Chen, L. -R., Huang, L. L. H., Shin, J. -W., & Lin, W. -C. (2017). Pathogenic Acanthamoeba castellanii Secretes the Extracellular Aminopeptidase M20/M25/M40 Family Protein to Target Cells for Phagocytosis by Disruption. Molecules, 22(12), 2263. https://doi.org/10.3390/molecules22122263