The Major Chromophore Arising from Glucose Degradation and Oxidative Stress Occurrence during Lens Proteins Glycation Induced by Glucose
Abstract
:1. Introduction
2. Results
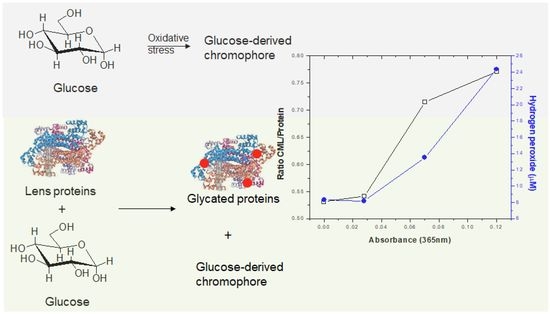
2.1. Effect of Oxidative and Glycoxidative Stress in the Generation of the Major Chromophore Arising from Glucose Degradation
2.2. Glucose Oxidized Derivatives Are Present in Enriched Fractions Containing the Major Chromophore Arising from Glucose Degradation
2.3. The Major Chromophore Arising from Glucose Degradation Generation Is Associated with Oxidative and Glycoxidative Modifications in Lens Proteins during Protein Glycation
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Isolation of Water-Soluble Bovine Lens Proteins
4.3. Evaluation of Oxidative Glycoxidative Stress in the Generation of the Major Chromophore Arising from Glucose Degradation
4.4. Generation and Purification of the Major Chromophore Arising from Glucose Degradation
4.5. Nuclear Magnetic Resonance (NMR) Analyses
4.6. FT-IR
4.7. Immunodetection and Quantification of Carboxymethyl-Lysine
4.8. Peroxides Quantification
4.9. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weikel, K.A.; Garber, C.; Baburins, A.; Taylor, A. Nutritional modulation of cataract. Nutr. Rev. 2014, 72, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.; Friguet, B.; Silva, E. Photosensitizing activity of endogenous eye lens chromophores: An attempt to unravel their contributions to photo-aging and cataract disease. Photochem. Photobiol. 2015, 91, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344 Pt 1, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Chetyrkin, S.; Mathis, M.; Pedchenko, V.; Sanchez, O.A.; McDonald, W.H.; Hachey, D.L.; Madu, H.; Stec, D.; Hudson, B.; Voziyan, P. Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry 2011, 50, 6102–6112. [Google Scholar] [CrossRef] [PubMed]
- Smuda, M.; Henning, C.; Raghavan, C.T.; Johar, K.; Vasavada, A.R.; Nagaraj, R.H.; Glomb, M.A. Comprehensive analysis of maillard protein modifications in human lenses: Effect of age and cataract. Biochemistry 2015, 54, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.; Trejo, S.; Baraibar, M.A.; Friguet, B.; Silva, E. Photosensitized reactions mediated by the major chromophore arising from glucose decomposition, result in oxidation and cross-linking of lens proteins and activation of the proteasome. Biochim. Biophys. Acta 2012, 1822, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Becker, M.I.; Friguet, B.; Silva, E.; Ávila, F. Oxidative modifications in crystallin proteins and lens epithelial cells associated with photosensitized reactions mediated by the major chromophore arising from glucose degradation. J. Braz. Chem. Soc. 2016, 27, 411–422. [Google Scholar] [CrossRef]
- Kamei, A. Glutathione levels of the human crystalline lens in aging and its antioxidant effect against the oxidation of lens proteins. Biol. Pharm. Bull. 1993, 16, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.H.; Truscott, R.J. An impediment to glutathione diffusion in older normal human lenses: A possible precondition for nuclear cataract. Exp. Eye Res. 1998, 67, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Kisic, B.; Miric, D.; Zoric, L.; Ilic, A.; Dragojevic, I. Antioxidant capacity of lenses with age-related cataract. Oxid. Med. Cell. Longev. 2012, 2012, 467130. [Google Scholar] [CrossRef] [PubMed]
- Viteri, G.; Carrard, G.; Birlouez-Aragon, I.; Silva, E.; Friguet, B. Age-dependent protein modifications and declining proteasome activity in the human lens. Arch. Biochem. Biophys. 2004, 427, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Linetsky, M.; Shipova, E.; Cheng, R.; Ortwerth, B.J. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochim. Biophys. Acta 2008, 1782, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Linstrom, J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2001. [CrossRef]
- Walker, T.E.; London, R.E.; Whaley, T.W.; Barker, R.; Matwiyoff, N.A. Carbon-13 nuclear magnetic resonance spectroscopy of [1-13C] enriched monosaccharides. Signal assignments and orientational dependence of geminal and vicinal carbon-carbon and carbon-hydrogen spin-spin coupling constants. J. Am. Chem. Soc. 1976, 98, 5807–5813. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. Hmdb 3.0—The human metabolome database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Rahman, M.A.; Salim, A.; Simjee, S.U. Advanced glycation end products in senile diabetic and nondiabetic patients with cataract. J. Diabetes Complicat. 2009, 23, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Haik, G.M., Jr.; Lo, T.W.; Thornalley, P.J. Methylglyoxal concentration and glyoxalase activities in the human lens. Exp. Eye Res. 1994, 59, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, E.; Andersson, D.K.; Stefansson, E. The prevalence of cataract in a population with and without type 2 diabetes mellitus. Acta Ophthalmol. 2012, 90, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Obrosova, I.G.; Chung, S.S.; Kador, P.F. Diabetic cataracts: Mechanisms and management. Diabetes Metab. Res. Rev. 2010, 26, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Wells-Knecht, K.J.; Zyzak, D.V.; Litchfield, J.E.; Thorpe, S.R.; Baynes, J.W. Mechanism of autoxidative glycosylation: Identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 1995, 34, 3702–3709. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification. The potential role of ′autoxidative glycosylation’ in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Saxena, O.C.; Singh, M.P. Mechanism of copper(II) oxidation of reducing sugars. I. Kinetics and mechanism of oxidation of d-xylose, l-arabinose, d-glucose, d-fructose, d-mannose, d-galactose, l-sorbose, lactose, maltose, cellobiose, and melibiose by copper(II) in alkaline medium. J. Am. Chem. Soc. 1970, 92, 537–541. [Google Scholar] [CrossRef]
- Spector, A.; Garner, W.H. Hydrogen peroxide and human cataract. Exp. Eye Res. 1981, 33, 673–681. [Google Scholar] [CrossRef]
- Deng, S.; Pizzi, A.; Du, G.; Zhang, J.; Zhang, J. Synthesis, structure, and characterization of glyoxal-urea-formaldehyde cocondensed resins. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Pecsok, R.L.; Juvet, R.S. The gluconate complexes. I. Copper gluconate complexes in strongly basic media1. J. Am. Chem. Soc. 1955, 77, 202–206. [Google Scholar] [CrossRef]
- Escandar, G.M.; Sala, L.F. Complexes of Cu(II) with d-aldonic and d-alduronic acids in aqueous solution. Can. J. Chem. 1992, 70, 2053–2057. [Google Scholar] [CrossRef]
- Abu-Bakr, M.S.; Rageh, H.M.; Hashem, E.Y.; Moustafa, M.H. Studies on the mixed-ligand complexes of Copper (II) with gallic acid and pyridine carboxyllic acids and their benzologues. Monatsh. Chem. 1994, 125, 1197–1205. [Google Scholar] [CrossRef]
- Suksrichavalit, T.; Prachayasittikul, S.; Piacham, T.; Isarankura-Na-Ayudhya, C.; Nantasenamat, C.; Prachayasittikul, V. Copper complexes of nicotinic-aromatic carboxylic acids as superoxide dismutase mimetics. Molecules 2008, 13, 3040–3056. [Google Scholar] [CrossRef] [PubMed]
- Kabir-ud, D.; Zaidi, N.H.; Akram, M.; Khan, Z. Mechanism of the oxidation of d-glucose onto colloidal MnO2 surface in the absence and presence of TX-100 micelles. Colloid Polym. Sci. 2006, 284, 1387–1393. [Google Scholar] [CrossRef]
- Abbadi, A.; van Bekkum, H. Effect of pH in the Pt-catalyzed oxidation of d-glucose to d-gluconic acid. J. Mol. Catal. A Chem. 1995, 97, 111–118. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Karam, A.; Trinh, Q.T.; Xu, K.; Hirao, H.; Jérôme, F.; Chatel, G. Selective and catalyst-free oxidation of d-glucose to d-glucuronic acid induced by high-frequency ultrasound. Sci. Rep. 2017, 7, 40650. [Google Scholar] [CrossRef] [PubMed]
- Meli, M.; Granouillet, O.R.; Reynaud, E.; Chamson l, A.; Frey, J.; Perier, C. Changes in glycation of fibrous type I collagen during long-term in vitro incubation with glucose. J. Protein Chem. 2003, 22, 521–525. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |









© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila, F.; Schmeda-Hirschmann, G.; Silva, E. The Major Chromophore Arising from Glucose Degradation and Oxidative Stress Occurrence during Lens Proteins Glycation Induced by Glucose. Molecules 2018, 23, 6. https://doi.org/10.3390/molecules23010006
Ávila F, Schmeda-Hirschmann G, Silva E. The Major Chromophore Arising from Glucose Degradation and Oxidative Stress Occurrence during Lens Proteins Glycation Induced by Glucose. Molecules. 2018; 23(1):6. https://doi.org/10.3390/molecules23010006
Chicago/Turabian StyleÁvila, Felipe, Guillermo Schmeda-Hirschmann, and Eduardo Silva. 2018. "The Major Chromophore Arising from Glucose Degradation and Oxidative Stress Occurrence during Lens Proteins Glycation Induced by Glucose" Molecules 23, no. 1: 6. https://doi.org/10.3390/molecules23010006
APA StyleÁvila, F., Schmeda-Hirschmann, G., & Silva, E. (2018). The Major Chromophore Arising from Glucose Degradation and Oxidative Stress Occurrence during Lens Proteins Glycation Induced by Glucose. Molecules, 23(1), 6. https://doi.org/10.3390/molecules23010006