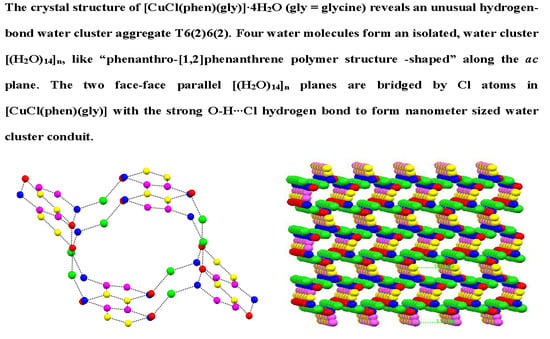
A Water Cluster Conduit in Crystal
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Desiraju, G.R. Hydration in organic crystals: Prediction from molecular structure. J. Chem. Soc. Chem. Commun. 1991, 6, 426–428. [Google Scholar] [CrossRef]
- Son, H.S.; Hong, B.H.; Lee, C.-W.; Yun, S.; Kim, K.S. A new type of helix pattern in polyalanine peptide. J. Am. Chem. Soc. 2001, 123, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Atwood, J.L.; Barbour, L.J.; Ness, T.J.; Raston, C.L.; Raston, P.L. A well-resolved ice-like (H2O)8 cluster in an organic supramolecular complex. J. Am. Chem. Soc. 2001, 123, 7192–7193. [Google Scholar] [CrossRef] [PubMed]
- Doedens, R.J.; Yohannes, E.; Khan, M.I. Novel water clusters in the crystalline state: Structures of a symmetrical, cyclic hexamer and an ‘opened-cube’ octamer. Chem. Commun. 2002, 1, 62–63. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Natarajan, R.; Venugopalan, P. Characterization of a planar cyclic form of water hexamer in an organic supramolecular complex: An unusual self-assembly of bimesityl-3,3’-dicarboxylic acid. Angew. Chem. Int. Ed. 2002, 41, 3417–3420. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bharadwaj, P.K. A dodecameric water cluster built around a cyclic quasiplanar hexameric core in an organic supramolecular complex of a cryptand. Angew. Chem. Int. Ed. 2004, 43, 3577–3580. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Xu, H.R.; Yang, C.F.; Wei, Z.H.; Zhang, N.; Huang, R.B.; Zheng, L.S. Encapsulated Diverse Water Aggregates in Two Ag(l)/4,4’-Bicarboxylate Hosts: 1D Water Tape and Chain. Cryst. Growth Des. 2010, 10, 4642–4649. [Google Scholar] [CrossRef]
- Luo, G.G.; Xiong, H.B.; Dai, J.C. Syntheses, Structural Characterization, and Properties of {[Cu(bPP)2(H2O)2](tp)center dot 7H2O} and {[Cu(bPP)2(H2O)](ip)center dot 7H2O} Complexes. New Examples of the ORGANIC anionic Template Effect on Induced Assembly of Water Clusters (bpp=1,3-Bis(4-pyridyl)propane,tp=Terephthalate,ip=isophthalate). Cryst. Growth Des. 2011, 11, 507–515. [Google Scholar]
- Luo, G.G.; Xiong, H.B.; Sun, D.; Wu, D.L.; Huang, R.B.; Dai, J.C. A Discrete spirocyclic (H2O)(9) Cluster and 1D Novel Water with Chain Tetrameric and Octameric Clusters in Cationic Hosts. Cryst. Growth Des. 2011, 11, 1948–1956. [Google Scholar] [CrossRef]
- Luo, G.G.; Wu, D.L.; Wu, J.H.; Xia, J.X.; Liu, L.; Dai, J.C. Direct observation of conformational change of adipate dianions encapsulated in water clusters. CrystEngComm 2012, 14, 5377–5380. [Google Scholar] [CrossRef]
- Buck, U.; Huisken, F. Infrared Spectroscopy of Size-Selected Water and Methanol Clusters. Chem. Rev. 2000, 100, 3863–3890. [Google Scholar] [CrossRef] [PubMed]
- Head-Gordon, T.; Hura, G. Water structure from scattering experiments and simulation. Chem. Rev. 2002, 102, 2651–2669. [Google Scholar] [CrossRef] [PubMed]
- Das, M.C.; Maity, S.B.; Bharadwaj, P.K. Supramolecular association of water molecules forming discrete clusters in the voids of coordination polymers. Curr. Opin. Solid State Mater. Sci. 2009, 13, 76–90. [Google Scholar] [CrossRef]
- Zuhayra, M.; Kampen, W.U.; Henze, E.; Soti, Z.; Zsolnai, L.; Huttner, G.; Oberdorfer, F. A planar water tetramer with tetrahedrally coordinated water embedded in a hydrogen bonding network of [Tc4(CO)12-(mu3-OH)4∙4H2O]. J. Am. Chem. Soc. 2006, 128, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Long, L.-S.; Wu, Y.-R.; Huang, R.-B.; Zheng, L.-S. A well-resolved uudd cyclic water tetramer in the crystal host of [Cu(adipate)(4,4-bipyridine)]∙(H2O)2. Inorg. Chem. 2004, 43, 3798–3800. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Sankaran, N.B.; Samanta, A. Structure of a self-assembled chain of water molecules in a crystal host. Angew. Chem. Int. Ed. 2003, 42, 1741–1743. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Nangia, A. First example of an ice-like water hexamer boat tape structure in a supramolecular organic host. Chem. Commun. 2006, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Custelcean, R.; Afloroaei, C.; Vlassa, M.; Polverejan, M. Formation of Extended Tapes of Cyclic Water Hexamers in an Organic Molecular Crystal Host. Angew. Chem. Int. Ed. 2000, 39, 3094–3096. [Google Scholar] [CrossRef]
- Ye, B.-H.; Ding, B.-B.; Weng, Y.-Q.; Chen, X.-M. Formation of one-dimensional metal-water chain containing cyclic water hexamers. Inorg. Chem. 2004, 43, 6866–6868. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.K. Coexistence of Water Dimer and Hexamer Clusters in 3D Metal-Organic Framework Structures of Ce(III) and Pr(III) with Pyridine-2,6-dicarboxylic Acid. Inorg. Chem. 2003, 42, 8250–8254. [Google Scholar]
- Mukhopadhyay, U.; Bernal, I. Self-Assembled Hexameric Water Clusters Stabilized by a Cyano-Bridged Trimetallic Complex. Cryst. Growth Des. 2005, 5, 1687–1689. [Google Scholar] [CrossRef]
- Tadokoro, M.; Fukui, S.; Kitajima, T.; Nagao, Y.; Ishimaru, S.; Kitagawa, H.; Isobe, K.; Nakasuji, K. Structures and phase transition of multi-layered water nanotube confined to nanochannels. Chem. Commun. 2006, 1274–1276. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.-Q.; Sun, H.-L.; Gao, S. Observation of an octameric water cluster containing a book-shaped hexamer in a 4f-3d complex. Chem. Commun. 2005, 2336–2338. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-C.; Jiang, Y.-C.; Wang, S.-L. Discrete water hexamers and template-assisted molecular recongnition in an elastic zincophosphate lattice. J. Am. Chem. Soc. 2005, 127, 12794–12795. [Google Scholar] [CrossRef] [PubMed]
- Keutsch, F.N.; Cruzan, J.D.; Saykally, R.J. The Water Trimer. Chem. Rev. 2003, 103, 2533–2578. [Google Scholar] [CrossRef] [PubMed]
- Carballo, R.; Covelo, B.; Lodeiro, C.; Vazquez-Lopez, E.M. One-dimensional fluorescent stacking structure based on zinc mixed-complex salt encapsulated within an ‘ice-like’ three-dimensional hydrogen-bonded water network. CrystEngComm 2005, 7, 294–296. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Vittal, J.J. Helix inside a Helix: Encapsulation of Hydrogen-Bonded Water Molecules in a Staircase Coordination Polymer. Angew. Chem. Int. Ed. 2004, 43, 5769–5772. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Jo, H.J.; Kim, J.; Kim, S.-Y.; Kim, D.; Kim, K. Supramolecular self-assembly of tin(IV) porphyrin channels stabilizing single-file chains of water molecules. CrystEngComm 2005, 7, 417–420. [Google Scholar] [CrossRef]
- Cheruzel, L.E.; Pometun, M.S.; Cecil, M.R.; Mashuta, M.S.; Wittebort, R.J.; Buchanan, R.M. Structures and Solid-State Dynamics of One-Dimensional Water Chains Stabilized by Imidazole Channels. Angew. Chem. Int. Ed. 2003, 42, 5452–5455. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Saha, M.K.; Nethaji, M.; Chakravarty, A.R. Helical supramolecular host with aquapores anchoring alternate molecules of helical water chains. Chem. Commun. 2004, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Neogi, S.; Bharadwaj, P.K. An Infinite Water Chain Passes through an Array of Zn(II) Metallocycles Built with a Podand Bearing Terminal Carboxylates. Inorg. Chem. 2005, 44, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-Y.; Xu, L. (H2O)12-containing infinite chain encapsulated in supramolecular open framework built of cadmium(II), 1,3-di(4-pyridyl)propane and 5-sulfoisophthalic acid monosodium salt. CrystEngComm 2005, 7, 87–89. [Google Scholar] [CrossRef]
- Assaf, K.I.; Ural, M.S.; Pan, F.F.; Georgiev, T.; Simova, S.; Rissanen, K.; Gabel, D.; Nau, W.M. Water Structure Recovery in Chaotropic Anion Recognition: High-Affinity Binding of Dodecaborate Clusters to γ-Cyclodextrin. Angew. Chem. Int. Ed. 2015, 54, 6956–6960. [Google Scholar] [CrossRef]
- Neogi, S.; Savitha, G.; Bharadwaj, P.K. Structure of Discrete (H2O)12 Clusters Present in the Cavity of Polymeric Interlinked Metallocycles of Nd(III) or Gd(III) and a Podand Ligand. Inorg. Chem. 2004, 43, 3771–3773. [Google Scholar] [CrossRef] [PubMed]
- Infantes, L.; Chisholm, J.; Motherwell, S. Extended motifs from water and chemical functional groups in organic molecular crystals. CrystEngComm 2003, 5, 480–486. [Google Scholar] [CrossRef]
- Infantes, L.; Motherwell, S. Water clusters in organic molecular crystals. CrystEngComm 2002, 4, 454–461. [Google Scholar] [CrossRef]
- Mascal, M.; Infantes, L.; Chisholm, J. Water Oligomers in Crystal Hydrates—What’s News and What Isn’t? Angew. Chem. Int. Ed. 2006, 45, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Kauzmann, W. The Structure and Properties of Water; Oxford University Press: New York, NY, USA, 1969. [Google Scholar]
- Banerjee, S.; Murugavel, R. Formation of One-Dimensional Water Inside an Organic Solid: Supramolecular Architectures Derived by the Interaction of Aminobenzoic Acids with Nitrogen Bases and H2SO4. Cryst. Growth Des. 2004, 4, 545–552. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound [CuCl(phen)(H2NCH2COO)]∙4H2O are available from the authors. |



| Length | Angle | ||
|---|---|---|---|
| O1w-O1 | 2.920(1) | O2w F…O1w…O3w C | 114.39 |
| O1w-O2 | 3.005(1) | O2w F…O1w…O1 | 84.88 |
| O1w-Cl(1) A | 3.080(2) | O2w F…O1w…O2 | 109.56 |
| O1w-O3w C | 2.757(1) | O2w F…O1w…Cl(1) A | 99.34 |
| O2w-O1w B | 2.743(1) | O3w C…O1w…O1 | 96.28 |
| O2w-O4w C | 2.754(1) | O3w C…O1w…O2 | 115.53 |
| N3-O2w C | 3.043 | O3w C…O1w…Cl(1) A | 126.05 |
| O2w B-Cl(1) | 3.223(1) | O1w B…O2w…O4w C | 98.83 |
| O3w-O4w | 2.403(1) | O1w B…O2w…N3 C | 94.22 |
| O3w-O3w D | 2.412(1) | O1w B…O2w…Cl(1) B | 103.79 |
| O4w-O4w E | 2.608(1) | O4w C…O2w…N3 C | 91.39 |
| Ring(i)→Ring(j)/C | Distance between the (i,j) Ring Centroids (Å) | Dihedral Angle (i,j) (Deg) | Distance of Centroid (i) from Ring (j) (Å) |
|---|---|---|---|
| R1→R4 i | 3.528 | 0.67 | 3.354 |
| R2→R3 | 3.618 | 2.10 | 3.379 |
| R2→R4 ii | 3.844 | 0.92 | 3.372 |
| R2→R4 i | 3.588 | 0.92 | 3.379 |
| R4→R4 ii | 3.528 | 0.67 | 3.389 |
| R4→R4 i | 3.865 | 0.00 | 3.389 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, F.; Liu, E.; Xu, J. A Water Cluster Conduit in Crystal. Molecules 2017, 22, 2278. https://doi.org/10.3390/molecules22122278
Jian F, Liu E, Xu J. A Water Cluster Conduit in Crystal. Molecules. 2017; 22(12):2278. https://doi.org/10.3390/molecules22122278
Chicago/Turabian StyleJian, Fangfang, E Liu, and Jiao Xu. 2017. "A Water Cluster Conduit in Crystal" Molecules 22, no. 12: 2278. https://doi.org/10.3390/molecules22122278
APA StyleJian, F., Liu, E., & Xu, J. (2017). A Water Cluster Conduit in Crystal. Molecules, 22(12), 2278. https://doi.org/10.3390/molecules22122278