Labdane-Type Diterpenes, Galangalditerpenes A–C, with Melanogenesis Inhibitory Activity from the Fruit of Alpinia galanga
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation
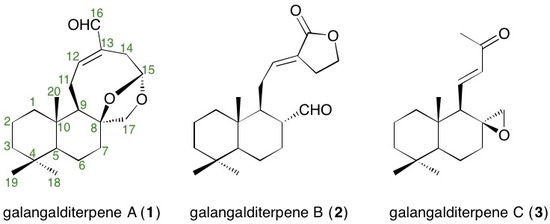
2.2. Structures of Galangalditerpenes A–C (1–3)
2.3. Effects on Theophylline-Stimulated Melanogenesis Inhibitory Activity
2.4. Effects on Mushroom Tyrosinase
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Conversion of Galanolactone (8) into Galangalditerpene B (2)
3.5. Hydrogenation of Galangalditerpene C (3)
3.6. Reagents for Bioassays
3.7. Cell Culture
3.8. Melanogenesis and Cell Viability
3.9. Mushroom Tyrosinase
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demetzos, C.; Dimas, K.S. Labdane-type diterpenes: Chemistry and biological activity. Stud. Nat. Prod. Chem. 2001, 25, 235–292. [Google Scholar]
- Chinou, I. Labdanes of natural origin-biological activities (1981–2004). Curr. Med. Chem. 2005, 12, 1295–1317. [Google Scholar] [CrossRef] [PubMed]
- Raviraja, S.G.; Monisha, S. Pharmacology of an endangered medicinal plant Alpinia galanga—A review. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 499–511. [Google Scholar]
- Matsui, S.; Kobayashi, S.; Nagahori, H.; Ogiso, A. Constituents from seeds of Alpinia galanga Wild. And their anti-ulcer activities. Chem. Pharm. Bull. 1976, 24, 2377–2382. [Google Scholar] [CrossRef]
- Morita, H.; Itokawa, H. New diterpenes from Alpinia galanga Wild. Chem. Lett. 1986, 15, 1205–1208. [Google Scholar] [CrossRef]
- Itokawa, H.; Morita, H.; Sumitomo, T.; Totsuka, N.; Takeya, K. Antitumor principles from Alpinia galanga. Planta Med. 1987, 53, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Itokawa, H. Cytotoxic and antifungal diterpenes from the seeds of Alpinia galanga. Planta Med. 1988, 54, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.L.; Zhou, K.Y.; Dong, H.; Xu, L.S. Characters of nrDNA ITS region sequences of fruits of Alpinia galanga and their adulterants. Planta Med. 2001, 67, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.-Q.; Kang, J.; Wang, H.-Q.; Zhang, Q.-J.; Liu, C.; Chen, R.-Y. Three new norsesquiterpenoids from the seeds of Alpinia galanga. J. Asian Nat. Prod. Res. 2014, 16, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.-H.; Lu, C.-L.; Zhang, X.-W.; Jiang, J.-G. Isolation and identification of ingredients inducing cancer cell death from the seeds of Alpinia galanga, a Chinese spice. Food Funct. 2015, 6, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Ochi, M.; Yoshikawa, M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: Structural requirements and mode of action. Eur. J. Pharmacol. 2003, 471, 59–67. [Google Scholar] [CrossRef]
- Morikawa, T.; Ando, S.; Matsuda, H.; Kataoka, S.; Muraoka, O.; Yoshikawa, M. Inhibitors of nitric oxide production from the rhizomes of Alpinia galanga: Structures of new 8–9′ linked neolignans and sesquineoliganns. Chem. Pharm. Bull. 2005, 53, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Kamei, I.; Katsuyama, Y.; Imagawa, T.; Chaipech, S.; Muraoka, O.; Morikawa, T. Melanogenesis inhibitory activity of a 7-O-9′-linked neolignan from Alpinia galanga fruit. Bioorg. Med. Chem. 2016, 24, 6215–6224. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Managi, H.; Yoshikawa, M. Antiallergic principles from Alpinia galanga: Structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. Lett. 2003, 13, 3197–3202. [Google Scholar] [CrossRef]
- Matsuda, H.; Ando, S.; Morikawa, T.; Kataoka, S.; Yoshikawa, M. Structure-activity relationships of 1′S-1′-acetoxychavicol acetate for inhibitory effect on NO production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. Lett. 2005, 15, 1949–1953. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Matsuda, H.; Morikawa, T.; Yoshikawa, M. 1′S-1′-acetoxtchavicol acetate as a new type inhibitor of interferon-β production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg. Med. Chem. 2005, 13, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Heymann, H.; Tezuka, Y.; Kikuchi, T.; Supriyatna, S. Constituents of Sindora sumatrana MIQ. I. Isolation and NMR spectral analysis of sesquiterpenes from the dried pods. Chem. Pharm. Bull. 1994, 42, 138–146. [Google Scholar] [CrossRef]
- Hansson, T.; Wickberg, B. A short enantiospecific route to isodaucane sesquiterpenes from limonene. On the absolute configuration of (+)-aphannamol I and II. J. Org. Chem. 1992, 57, 5370–5376. [Google Scholar] [CrossRef]
- Itokawa, H.; Yoshimoto, S.; Morita, H. Diterpenes from the rhizomes of Alpinia formosana. Phytochemistry 1988, 27, 435–438. [Google Scholar] [CrossRef]
- Jung, M.; Lee, S.; Yoon, B. Conversion of sclareol into (+)-galanolactone and (+)-labdienedial. Tetrahedron Lett. 1997, 38, 2871–2874. [Google Scholar] [CrossRef]
- Winter, B. Spirocyclic ethers related to Ambrox®: Synthesis and structure-odor relationships. Helv. Chim. Acta 2004, 87, 1616–1627. [Google Scholar] [CrossRef]
- Grant, P.K.; Weavers, R.T. Diterpene chemistry—IV transformations of 8,(17)-labdadien-13-ol. Tetrahedron 1974, 30, 2385–2395. [Google Scholar] [CrossRef]
- Sajiki, H.; Hattori, K.; Hirota, K. Highly chemoselective hydrogenation with retention of the epoxide function using a heterogeneous Pd/C-ethylenediamine catalyst and THF. Chem. Eur. J. 2000, 6, 2200–2204. [Google Scholar] [CrossRef]
- Morikawa, T.; Nakanishi, Y.; Ninomiya, K.; Matsuda, H.; Nakashima, S.; Miki, H.; Miyashita, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med. 2104, 68, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, G.; Sugano, Y.; Shirato, M.; Sonoda, N.; Tsutsui, N.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Muraoka, O. Total synthesis of 4,5-didehydroguadiscine: A potent melanogenesis inhibitor from the Brazilian medicinal herb, Hornschuchia obliqua. J. Nat. Prod. 2015, 78, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ninomiya, K.; Kuramoto, H.; Kamei, I.; Yoshikawa, M.; Muraoka, O. Phenylethanoid and phenylpropanoid glycosides with melanogenesis inhibitory activity from the flowers of Narcissus tazetta var. chinensis. J. Nat. Med. 2016, 70, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Matsumoto, T.; Chaipech, S.; Miyake, S.; Katsuyama, Y.; Tsuboyama, A.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O.; Morikawa, T. Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora. J. Nat. Med. 2016, 70, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Kitagawa, N.; Tanabe, G.; Ninomiya, K.; Okugawa, S.; Motai, C.; Kamei, I.; Yoshikawa, M.; Lee, I.J.; Muraoka, O. Quantitative determination of alkaloids in lotus flower (flower buds of Nelumbo nucifera) and their melanogenesis inhibitory activity. Molecules 2016, 21, 930. [Google Scholar] [CrossRef] [PubMed]
- Manse, Y.; Ninomiya, K.; Okazaki, A.; Okada-Nishida, E.; Imagawa, T.; Imamura-Mizushima, M.; Yamano, Y.; Kaname, K.; Nakamura, S.; Morikawa, T. Melanogenesis inhibitory activity of diterpenoid and triterpenoid constituents from the aerial part of Isodon trichocarpus. Nat. Prod. Commun. 2017, 12, 1185–1188. [Google Scholar]
Sample Availability: Samples of the compounds 1–9 are available from the authors. |





| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 1.04 (ddd, 3.2, 12.8, 12.8, Hα), 1.87 (ddd, 2.4, 3.2, 12.8, Hβ) | 39.4 | 1.02 (ddd, 4.0, 12.8, 12.8, Hα), 1.76 (m, Hβ) | 39.0 | 0.96 (ddd, 3.6, 12.8, 12.8, Hα), 1.42 (m, Hβ) | 40.5 |
| 2 | 1.60 (m, Hα), 1.51 (ddddd, 2.4, 3.2, 12.8, 13.6, 14.4, Hβ) | 18.6 | 1.76 (ddddd, 2.4, 3.2, 4.0, 4.0, 14.4, Hα), 1.50 (ddddd, 3.6, 4.0, 12.8, 13.4, 14.4, Hβ) | 18.5 | 1.51 (m, Hα), 1.41 (m, Hβ) | 18.3 |
| 3 | 1.19 (ddd, 4.0, 13.6, 13.6, Hα), 1.41 (ddd, 2.4, 2.4, 13.6, Hβ) | 41.6 | 1.20 (ddd, 4.0, 13.4, 13.4, Hα), 1.76 (ddd, 2.4, 3.6, 13.4, Hβ) | 41.8 | 1.17 (ddd, 3.2, 13.6, 13.6, Hα), 1.43 (m, Hβ) | 41.9 |
| 4 | 33.4 | 33.3 | 33.5 | |||
| 5 | 0.96 (dd, 2.4, 12.8) | 55.5 | 0.96 (dd, 2.4, 12.4) | 54.5 | 1.00 (dd, 2.4, 12.0) | 54.2 |
| 6 | 1.74 (dddd, 2.4, 4.4, 4.4, 13.6, Hα), 1.15 (dddd, 2.4, 3.2, 12.8, 13.6, Hβ) | 20.5 | 1.74 (m, Hα), 1.35 (dddd, 4.0, 12.0, 12.4, 12.4, Hβ) | 20.0 | 1.71 (br ddd, ca. 2, 6, 14, Hα), 1.66 (dddd, 4.0, 12.0, 13.6, 14.4, Hβ) | 19.9 |
| 7 | 1.64 (m, Hα), 1.84 (ddd, 2.4, 3.2, 12.8, Hβ) | 40.1 | 1.40 (dddd, 4.0, 12.0, 12.4, 12.4, Hα), 1.74 (m, Hβ) | 26.8 | 1.97 (ddd, 5.6, 13.6, 14.4, Hα), 1.42 (m, Hβ) | 18.3 |
| 8 | 84.7 | 2.28 (dddd, 3.2, 5.6, 12.0, 14.4) | 53.8 | 58.2 | ||
| 9 | 1.64 (m) | 57.4 | 1.54 (ddd, 3.2, 6.4, 14.4) | 50.3 | 1.42 (br d, ca. 10) | 56.9 |
| 10 | 39.5 | 37.5 | 39.5 | |||
| 11 | 2.40 (br dd, ca. 9, 13, Hα), 2.57 (ddd, 8.8, 10.4, 12.8, Hβ) | 24.7 | 1.81 (ddd, 6.4, 9.6, 12.8), 2.40 (ddd, 3.2, 6.4, 12.8) | 29.9 | 6.49 (dd, 10.4, 16.8) | 144.4 |
| 12 | 6.87 (ddd, 2.4, 8.8, 8.8) | 157.6 | 6.62 (dd, 6.4, 9.6) | 139.6 | 6.01 (d, 16.8) | 135.8 |
| 13 | 140.4 | 126.2 | 198.4 | |||
| 14 | 2.27 (ddd, 2.4, 3.2, 16.0, Hα), 3.13 (dd, 5.6, 16.0, Hβ) | 29.6 | 2.70 (m), 2.74 (m) | 25.3 | ||
| 15 | 5.44 (dd, 3.2, 5.6) | 100.4 | 4.34 (ddd, 6.4, 7.2, 14.4), 4.37 (ddd, 6.4, 7.6, 14.4) | 65.4 | ||
| 16 | 9.35 (s) | 194.1 | 171.1 | 2.23 (3H, s) | 26.4 | |
| 17 | 3.62 (d, 9.6, Hα), 4.35 (d, 9.6, Hβ) | 70.4 | 9.28 (d, 5.6) | 203.8 | 2.31 (d, 4.8, Hα), 2.38 (d, 4.8, Hβ) | 48.8 |
| 18 | 0.80 (3H, s) | 21.5 | 0.84 (3H, s) | 21.7 | 0.90 (3H, s) | 21.9 |
| 19 | 0.89 (3H, s) | 33.5 | 0.89 (3H, s) | 33.4 | 0.91 (3H, s) | 33.5 |
| 20 | 0.81 (3H, s) | 14.9 | 0.87 (3H, s) | 14.2 | 1.09 (3H, s) | 15.6 |
| Treatment | Inhibition (%) | IC50 (µM) | ||||
|---|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | ||
| Galangalditerpene A (1) | 0.0 ± 4.6 (100.0 ± 1.3) | 48.3 ± 4.1 ** (95.1 ± 0.9) | 56.6 ± 6.9 ** (91.4 ± 1.9) | 67.9 ± 5.9 ** (78.4 ± 2.3 #) | 86.2 ± 4.4 ** (48.6 ± 1.1 #) | 4.4 |
| Galangalditerpene B (2) | 0.0 ± 9.3 (100.0 ± 3.9) | 30.5 ± 9.1 ** (94.2 ± 6.1) | 50.9 ± 3.4 ** (107.3 ± 3.9) | 80.6 ± 2.3 ** (137.1 ± 1.6) | 85.3 ± 2.6 ** (126.5 ± 1.7) | 8.6 |
| Galangalditerpene C (3) | 0.0 ± 9.5 (100.0 ± 6.0) | 40.6 ± 2.8 ** (94.2 ± 3.7) | 67.1 ± 2.2 ** (114.9 ± 1.8) | 82.5 ± 4.4 ** (126.9 ± 4.1) | — (13.1 ± 0.6 #) | 4.6 |
| Clovane-2β,9α-diol (4) | 0.0 ± 3.4 (100.0 ± 4.7) | 23.9 ± 3.9 ** (98.1 ± 3.0) | 37.2 ± 2.6 ** (93.8 ± 1.4) | 64.0 ± 4.7 ** (102.3 ± 3.2) | 73.6 ± 3.7 ** (76.2 ± 3.6 #) | 17.7 |
| Caryolane-1,9β-diol (5) | 0.0 ± 6.0 (100.0 ± 4.2) | 37.3 ± 3.2 ** (98.4 ± 3.3) | 53.8 ± 2.0 ** (98.2 ± 2.5) | 52.7 ± 7.3 ** (105.3 ± 1.5) | 87.9 ± 3.5 ** (96.7 ± 1.1) | 9.4 |
| (−)-2-Oxoisodauc-5-en-12-al (6) | 0.0 ± 11.8 (100.0 ± 4.2) | 45.9 ± 7.8 ** (87.7 ± 2.4) | 67.8 ± 3.3 ** (86.3 ± 2.6) | 85.1 ± 2.2 ** (90.0 ± 0.8) | 86.7 ± 4.0 ** (59.9 ± 0.3 #) | 2.9 |
| Kobusone (7) | 0.0 ± 6.6 (100.0 ± 1.8) | −16.1 ± 3.6 (97.0 ± 2.1) | 20.5 ± 8.3 * (97.3 ± 2.2) | 51.5 ± 7.5 ** (92.1 ± 1.4) | 82.8 ± 2.7 ** (87.5 ± 0.5) | 29.8 |
| Galanolactone (8) | 0.0 ± 9.4 (100.0 ± 5.6) | 38.6 ± 4.1 ** (95.3 ± 5.2) | 64.4 ± 7.5 ** (113.6 ± 4.1) | 83.8 ± 3.0 ** (134.4 ± 4.1) | — (39.9 ± 3.6 #) | 5.2 |
| (E)-15,16-Bisnorlabda-8(17), 11-diene-13-one (9) | 0.0 ± 5.8 (100.0 ± 2.3) | 56.8 ± 3.4 ** (91.4 ± 1.3) | 60.8 ± 2.2 ** (98.5 ± 1.6) | 71.1 ± 1.4 ** (107.3 ± 2.0) | — (36.4 ± 1.6 #) | 2.0 |
| Arbutin [13,24,25,26,27,28,29] | 0.0 ± 1.4 (100.0 ± 2.1) | 20.4 ± 0.5 (82.4 ± 3.0) | 38.1 ± 0.9 ** (78.1 ± 1.9) | 61.5 ± 0.6 ** (79.8 ± 2.2) | 83.7 ± 0.5 ** (53.1 ± 1.8 #) | 174 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manse, Y.; Ninomiya, K.; Nishi, R.; Hashimoto, Y.; Chaipech, S.; Muraoka, O.; Morikawa, T. Labdane-Type Diterpenes, Galangalditerpenes A–C, with Melanogenesis Inhibitory Activity from the Fruit of Alpinia galanga. Molecules 2017, 22, 2279. https://doi.org/10.3390/molecules22122279
Manse Y, Ninomiya K, Nishi R, Hashimoto Y, Chaipech S, Muraoka O, Morikawa T. Labdane-Type Diterpenes, Galangalditerpenes A–C, with Melanogenesis Inhibitory Activity from the Fruit of Alpinia galanga. Molecules. 2017; 22(12):2279. https://doi.org/10.3390/molecules22122279
Chicago/Turabian StyleManse, Yoshiaki, Kiyofumi Ninomiya, Ryosuke Nishi, Yoshinori Hashimoto, Saowanee Chaipech, Osamu Muraoka, and Toshio Morikawa. 2017. "Labdane-Type Diterpenes, Galangalditerpenes A–C, with Melanogenesis Inhibitory Activity from the Fruit of Alpinia galanga" Molecules 22, no. 12: 2279. https://doi.org/10.3390/molecules22122279
APA StyleManse, Y., Ninomiya, K., Nishi, R., Hashimoto, Y., Chaipech, S., Muraoka, O., & Morikawa, T. (2017). Labdane-Type Diterpenes, Galangalditerpenes A–C, with Melanogenesis Inhibitory Activity from the Fruit of Alpinia galanga. Molecules, 22(12), 2279. https://doi.org/10.3390/molecules22122279