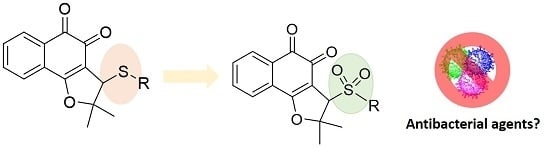
Efficient Catalytic Oxidation of 3-Arylthio- and 3-Cyclohexylthio-lapachone Derivatives to New Sulfonyl Derivatives and Evaluation of Their Antibacterial Activities
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Sulfonyl-nor-β-lapachone Derivatives 7a–g
2.2. Antibacterial Evaluation of Polyvinylpyrrolidone (PVP) Formulations of 3-Arylthio-nor-β-lapachone Derivatives 4a–f, 3-Cyclohexylthio-nor-β-lapachone 4g and of the Corresponding Sulfonyl Nor-β-lapachone Derivatives 7a–g
2.2.1. Incorporation of 3-Arylthio/Cyclohexylthio-nor-β-lapachone Derivatives 4a–g and Nor-β-lapachone Derivatives 7a–g into Polyvinylpyrrolidone Micelles
2.2.2. Antibacterial Evaluation of PVP Formulations of 4a–g and 7a–g
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of 3-Cyclohexylthio-nor-β-lapachone (4g)
3.3. General Procedure for the Synthesis of 3-Aryl/Cyclohexyl-sulfonyl-nor-β-lapachone Derivatives (7a–g)
3.4. General Procedure for the Incorporation of 3-Arylthio/cyclohexylthio-nor-β-lapachone Derivatives 4a–g and 3-Aryl/Cyclohexyl-sulfonyl-nor-β-lapachone 7a–g Derivatives into PVP Micelles
3.5. Antibacterial Evaluation of PVP Formulations of 3-Arylthio/Cyclohexylthio-nor-β-lapachone Derivatives 4a–g and 3-sulfonyl-nor-β-lapachones 7a–g
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferreira, S.B.C.; da Silva, F.C.; Bezerra, F.A.F.M.; Lourenço, M.C.S.; Kaiser, C.R.; Pinto, A.C.; Ferreira, V.F. Synthesis of α- and β-pyran naphthoquinones as a new class of antitubercular agents. Arch. Pharm. Chem. Life Sci. 2010, 343, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, S.; Li, J.; Chen, Y.; Saurav, K.; Zhang, Q.; Zhang, H.; Zhang, W.; Zhang, W.; Zhang, S.; et al. Antibacterial and cytotoxic new napyradiomycins from the marine-derived Streptomyces sp. SCSIO 10428. Mar. Drugs 2013, 11, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.; Johann, S.; Lima, L.A.R.S.; Campos, F.F.; Mendes, I.C.; Beraldo, H.; de Souza-Fagundes, E.M.; Cisalpino, P.S.; Rosa, C.A.; Alves, T.M.A.; et al. The antimicrobial activity of lapachol and its thiosemicarbazone and semicarbazone derivatives. Mem. Inst. Oswaldo Cruz 2013, 108, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, P.; Steiman, R.; Campos-Takaki, G.M.; Seigle-Murandi, E.; Simeon, B.M. Comparison of antibacterial and antifungal activities of lapachol and β-lapachone. Planta Med. 1994, 60, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.V.; Gilbert, B.; Pinto, M.C. In vitro and in vivo evaluation of the toxicity of 1,4-naphthoquinone and 1,2-naphthoquinone derivatives against Trypanosoma cruzi. Ann. Trop. Med. Parasitol. 1978, 72, 523–531. [Google Scholar]
- Corrêa, G.; Vilela, R.; Menna-Barreto, R.F.S.; Midlej, V.; Benchimol, M. Cell death induction in Giardia lamblia: Effect of β-lapachone and starvation. Parasitol. Int. 2009, 58, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.B.; Amorim, R.; da Silva, F.C.; Rocha, D.R.; Papa, M.P.; Arruda, L.B.; Borges, R.S.M.; Ferreira, V.F.; Tanuri, A.; Costa, L.J.; et al. Synthetic 1,4-pyran naphthoquinones are potent inhibitors of dengue virus replication. PLoS ONE 2013, 8, e82504. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Villamil, S.H.; Carrizo, P.H.; di Rosso, M.E.; Molina-Portela, M.P.; Dubin, M. The metabolism of 9-chloro-β-lapachone and its effects in isolated hepatocytes. The involvement of NAD(P)H:quinone oxidoreductase 1 (NQO1). Chem. Biol. Interact. 2012, 200, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Tang, Y.L.; Zhang, Z.H.; Liu, C.J.; Li, H.Z.; Li, R.T.; Xia, X.S. Compounds from Arnebia euchroma and their related anti-HCV and antibacterial activities. Planta Med. 2012, 78, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Crosby, I.T.; Bourke, D.G.; Jones, E.D.; Jeynes, T.P.; Cox, S.; Coates, J.A.; Robertson, A.D. Antiviral agents 3. Discovery of a novel small molecule non-nucleoside inhibitor of Hepatitis B Virus (HBV). Bioorg. Med. Chem. Lett. 2011, 21, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, B.C.; Barros, F.W.A.; Cabral, I.O.; Ferreira, J.R.O.; Magalhães, H.I.F.; Júnior, H.V.N.; da Silva Júnior, E.N.; de Abreu, F.C.; Costa, C.O.; Goulart, M.O.F.; et al. Preclinical genotoxicology of nor-β-lapachone in human cultured lymphocytes and Chinese hamster lung fibroblasts. Chem. Res. Toxicol. 2011, 24, 1560–1574. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.F.; Ferreira, S.B.; da Silva, F.C. Strategies for the synthesis of bioactive pyran naphthoquinones. Org. Biomol. Chem. 2010, 8, 4793–4802. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.N., Jr.; de Deus, C.F.; Cavalcanti, B.C.; Pessoa, C.; Costa-Lotufo, L.V.; Montenegro, R.C.; de Moraes, M.O.; Pinto, M.C.F.R.; de Simone, C.A.; Ferreira, V.F.; et al. 3-arylamino and 3-alkoxy-nor-β-lapachone derivatives: synthesis and cytotoxicity against cancer cell lines. J. Med. Chem. 2010, 53, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Júnior, E.N.; de Moura, M.A.B.F.; Pinto, A.V.; Pinto, M.C.F.R.; de Souza, M.C.B.V.; Araújo, A.J.; Pessoa, C.; Costa-Lotufo, L.V.; Montenegro, R.C.; de Moraes, M.O.; et al. Cytotoxic, trypanocidal activities and physicochemical parameters of nor-β-lapachone-based 1,2,3-triazoles. J. Braz. Chem. Soc. 2009, 20, 635–643. [Google Scholar] [CrossRef]
- Da Silva, E.N., Jr.; de Souza, M.C.B.V.; Fernandes, M.C.; Menna-Barreto, R.F.S.; Pinto, M.C.F.R.; Lopes, F.A.; de Simone, C.A.; Andrade, C.K.Z.; Pinto, A.V.; Ferreira, V.F.; et al. Synthesis and anti-Trypanosoma cruzi activity of derivatives from nor-lapachones and lapachones. Bioorg. Med. Chem. 2008, 16, 5030–5038. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.J.; Rumie Vittar, N.B.; da Silva, F.C.; Ferreira, V.F.; Rivarola, V.A. Synergistic enhancement of antitumor effect of β-lapachone by photodynamic induction of quinone oxidoreductase (NQO1). Phytomedicine 2013, 20, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.I.; Vargas, M.D.; Fragoso, T.P.; Carneiro, J.W.M.; Casellato, A.; da Silva, F.C.; Ferreira, V.F.; Barbosa, J.P.; Pessoa, C.O.; Lotufo, L.V.; et al. Theoretical studies of the tautomerism in 3-(2-R-phenylhydrazono)-naphthalene-1,2,4-triones: Synthesis of copper(II) complexes and studies of antibacterial and antitumor activities. J. Braz. Chem. Soc. 2010, 21, 1293–1302. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Cabral, I.O.; Rodrigues, F.A.R.; Barros, F.W.A.; Rocha, D.D.; Magalhães, H.I.F.; Moura, D.J.; Saffi, J.; Henriques, J.A.P.; Carvalho, T.S.C.; et al. Potent antileukemic action of naphthoquinoidal compounds: Evidence for an intrinsic death mechanism based on oxidative stress and inhibition of DNA repair. J. Braz. Chem. Soc. 2013, 24, 145–163. [Google Scholar] [CrossRef]
- Cruz, E.H.G.; Hussene, C.M.B.; Dias, G.G.; Diogo, E.B.T.; Melo, I.M.M.; Rodrigues, B.L.; Silva, M.G.; Valença, W.O.; Câmara, C.A.; Oliveira, R.N.; et al. 1,2,3-Triazole-, arylamino- and thio-substituted 1,4-naphthoquinones: Potent antitumor activity, electrochemical aspects, and bioisosteric replacement of C-ring-modified lapachones. Bioorg. Med. Chem. 2014, 22, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.F.C.; da Silva, I.M.C.B.; Santos, H.M., Jr.; Rocha, D.R.; Araújo, A.J.; Pessoa, C.; Moraes, M.O.; Costa-Lotufo, L.V.; da Silva, F.C.; Santos, W.C.; et al. A new approach for the synthesis of 3-substituted cytotoxic nor-β-lapachones. J. Braz. Chem. Soc. 2013, 24, 12–16. [Google Scholar] [CrossRef]
- Ferreira, F.R.; Ferreira, S.B.; Araújo, A.J.; Marinho Filho, J.D.B.; Pessoa, C.; Moraes, M.O.; Costa-Lotufo, L.V.; Montenegro, R.C.; da Silva, F.C.; Ferreira, V.F.; et al. Arylated α- and β-dihydrofuran naphthoquinones: Electrochemical parameters, evaluation of antitumor activity and their correlation. Electrochim. Acta 2013, 110, 634–640. [Google Scholar] [CrossRef]
- Da Silva, E.N., Jr.; de Souza, M.C.B.V.; Pinto, A.V.; Pinto, M.C.F.R.; Goulart, M.O.F.; Barros, F.W.A.; Pessoa, C.; Costa-Lotufo, L.V.; Montenegro, R.C.; de Moraes, M.O.; et al. Synthesis and potent antitumor activity of new arylamino derivatives of nor-β-lapachone and nor-α-lapachone. Bioorg. Med. Chem. 2007, 15, 7035–7041. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.B.; Salomão, K.; da Silva, F.C.; Pinto, A.V.; Kaiser, C.R.; Pinto, A.C.; Ferreira, V.F.; de Castro, S.L. Synthesis and anti-Trypanosoma cruzi activity of β-lapachone analogues. Eur. J. Med. Chem. 2011, 46, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C.; Ferreira, S.B.; Rocha, D.R.; Ferreira, V.F. Chagas disease: Challenges in developing new trypanocidal lead compounds. Rev. Virtual Quim. 2012, 4, 46–72. [Google Scholar] [CrossRef]
- Bourguignon, S.C.; Cavalcanti, D.F.B.; Souza, A.M.T.; Castro, H.C.; Rodrigues, C.R.; Albuquerque, M.G.; Santos, D.O.; Silva, G.G.; da Silva, F.C.; Ferreira, V.F.; et al. Trypanosoma cruzi: Insights into naphthoquinone effects on growth and proteinase activity. Exp. Parasitol. 2011, 127, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, S.C.; Castro, H.C.; Santos, D.O.; Alves, C.R.; Ferreira, V.F.; Gama, I.L.; da Silva, F.C.; Seguins, W.S.; Pinho, R.T. Trypanosoma cruzi: In vitro activity of epoxy-alpha-Lap, a derivative of alpha-lapachone, on trypomastigote and amastigote forms. Exp. Parasitol. 2009, 122, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.P.V.; Ferreira, S.B.; Oliveira, N.S.M.; Matsuura, A.B.J.; Gama, I.L.; da Silva, F.C.; Souza, M.C.B.V.; Lima, E.S.; Ferreira, V.F. Synthesis and biological evaluation of substituted α- and β-2,3-dihydrofuran naphthoquinones as potent anticandidal agents. Med. Chem. Commun. 2010, 1, 229–232. [Google Scholar] [CrossRef]
- Villar, R.; Encio, I.; Migliaccio, M.; Gil, M.J.; Martinez-Merino, V. Synthesis and cytotoxic activity of lipophilic sulphonamide derivatives of the benzo[b]thiophene 1,1-dioxide. Bioorg. Med. Chem. 2004, 12, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.F.C.; Salomão, K.; Bombaça, A.C.; da Rocha, D.R.; da Silva, F.C.; Cavaleiro, J.A.S.; Castro, S.L.; Ferreira, V.F. Synthesis and anti-Trypanosoma cruzi activity of new 3-phenylthio-nor-β-lapachone derivatives. Bioorg. Med. Chem. 2015, 23, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Shagufta. Sulfones: An important class of organic compounds with diverse biological activities. Int. J. Pharm. Pharm. Sci. 2015, 7, 19–27. [Google Scholar]
- Lee, K.; Cho, S.H.; Lee, J.H.; Goo, J.; Lee, S.Y.; Boovanahalli, S.K.; Yeo, S.K.; Lee, S.-J.; Kim, Y.K.; Kim, D.H.; et al. Synthesis of a novel series of 2-alkylthio substituted naphthoquinones as potent acyl-CoA: Cholesterol acyltransferase (ACAT) inhibitors. Eur. J. Med. Chem. 2013, 62, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, W.; Karst, U. Biomimetic modeling of oxidative drug metabolism. Anal. Bioanal. Chem. 2008, 391, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Bernadou, J.; Meunier, B. Biomimetic Chemical Catalysts in the Oxidative Activation of Drugs. Adv. Synth. Catal. 2004, 346, 171–184. [Google Scholar] [CrossRef]
- Mansuy, D. A brief history of the contribution of metalloporphyrin models to cytochrome P450 chemistry and oxidation catalysis. C. R. Chimie 2007, 10, 392–413. [Google Scholar] [CrossRef]
- Simões, M.M.Q.; Neves, C.M.B.; Pires, S.M.G.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. P450 Mimicking Processes and the Use of Metalloporphyrins. Pure Appl. Chem. 2013, 85, 1671–1681. [Google Scholar] [CrossRef]
- Martins, R.R.L.; Neves, M.G.P.M.S.; Silvestre, A.J.D.; Simões, M.M.Q.; Silva, A.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Tagliatesta, P.; Crestini, C. Oxidation of unsaturated monoterpenes with hydrogen peroxide catalysed by manganese(III) porphyrin complexes. J. Mol. Catal. A Chem. 2001, 172, 33–42. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Oxidation of alkylaromatics with hydrogen peroxide catalysed by manganese(III) porphyrins in the presence of ammonium acetate. J. Mol. Catal. A Chem. 2003, 201, 9–22. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Gonçalves, A.R.; Pereira, M.M.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Epoxidation reactions with hydrogen peroxide activated by a novel heterogeneous metalloporphyrin catalyst. J. Mol. Catal. A Chem. 2006, 256, 321–323. [Google Scholar] [CrossRef]
- Pires, S.M.G.; de Paula, R.; Simões, M.M.Q.; Silva, A.M.S.; Domingues, M.R.M.; Santos, I.C.M.S.; Vargas, M.D.; Ferreira, V.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Novel biomimetic oxidation of lapachol with H2O2 catalysed by a manganese(III) porphyrin complex. RSC Adv. 2011, 1, 1195–1199. [Google Scholar] [CrossRef]
- Pires, S.M.G.; Simões, M.M.Q.; Santos, I.C.M.S.; Rebelo, S.L.H.; Pereira, M.M.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Biomimetic oxidation of organosulfur compounds with hydrogen peroxide catalyzed by manganese porphyrins. Appl. Catal. A Gen. 2012, 439–440, 51–56. [Google Scholar] [CrossRef]
- Pires, S.M.G.; Simões, M.M.Q.; Santos, I.C.M.S.; Rebelo, S.L.H.; Paz, F.A.A.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Oxidation of organosulfur compounds using an Iron(III) porphyrin complex: An environmentally safe and efficient approach. Appl. Catal. B Environ. 2014, 160–161, 80–88. [Google Scholar] [CrossRef]
- Da Silva, G.; Pires, S.M.G.; Silva, V.L.M.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Rebelo, S.L.H.; Silva, A.M.S.; Cavaleiro, J.A.S. A green and sustainable method for the oxidation of 1,3-dihydrobenzo[c]thiophenes to sulfones using metalloporphyrin complexes. Catal. Commun. 2014, 56, 68–71. [Google Scholar] [CrossRef]
- Isakau, H.A.; Parkhats, M.V.; Knyukshto, V.N.; Dzhagarov, B.M.; Petrov, E.P.; Petrov, P.T. Toward understanding the high PDT efficacy of chlorin e6–polyvinylpyrrolidone formulations: Photophysical and molecular aspects of photosensitizer–polymer interaction in vitro. J. Photochem. Photobiol. B 2008, 92, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Schwach-Abdellaouia, K.; Vivien-Castionib, N.; Gurny, R. Local delivery of antimicrobial agents for the treatment of periodontal disease. Eur. J. Pharm. Biopharm. 2000, 50, 83–99. [Google Scholar] [CrossRef]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Springer-Verlag: Berlin, Germany, 2005. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
- Sample Availability: Samples of all compounds are available from the authors.





| Entry | Compounds 4 | η, Compounds 7 (%) |
|---|---|---|
| 1 | a | 84 |
| 2 | b | 86 |
| 3 | c | 81 |
| 4 | d | 85 |
| 5 | e | 80 |
| 6 | f | 78 |
| 7 | g | 81 |
| PVP Formulation (1 mM) | 4a | 4b | 4c | 4d | 4e | 4f | 4g | 7a | 7b | 7c | 7d | 7e | 7f | 7g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria S. aureus 2065 MA | 0 | 9 | 8 | 11 | 8 | 10 | 8 | 0 | 0 | 0 | 0 | 7 | 0 | 8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, M.F.d. C.; Gomes, A.T.P.C.; Moreira, C.D. S.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Da Rocha, D.R.; Da Silva, F.D.C.; Moreirinha, C.; Almeida, A.; Ferreira, V.F.; et al. Efficient Catalytic Oxidation of 3-Arylthio- and 3-Cyclohexylthio-lapachone Derivatives to New Sulfonyl Derivatives and Evaluation of Their Antibacterial Activities. Molecules 2017, 22, 302. https://doi.org/10.3390/molecules22020302
Cardoso MFdC, Gomes ATPC, Moreira CDS, Simões MMQ, Neves MGPMS, Da Rocha DR, Da Silva FDC, Moreirinha C, Almeida A, Ferreira VF, et al. Efficient Catalytic Oxidation of 3-Arylthio- and 3-Cyclohexylthio-lapachone Derivatives to New Sulfonyl Derivatives and Evaluation of Their Antibacterial Activities. Molecules. 2017; 22(2):302. https://doi.org/10.3390/molecules22020302
Chicago/Turabian StyleCardoso, Mariana F. do C., Ana T. P. C. Gomes, Caroline Dos S. Moreira, Mário M. Q. Simões, Maria G. P. M. S. Neves, David R. Da Rocha, Fernando De C. Da Silva, Catarina Moreirinha, Adelaide Almeida, Vitor F. Ferreira, and et al. 2017. "Efficient Catalytic Oxidation of 3-Arylthio- and 3-Cyclohexylthio-lapachone Derivatives to New Sulfonyl Derivatives and Evaluation of Their Antibacterial Activities" Molecules 22, no. 2: 302. https://doi.org/10.3390/molecules22020302
APA StyleCardoso, M. F. d. C., Gomes, A. T. P. C., Moreira, C. D. S., Simões, M. M. Q., Neves, M. G. P. M. S., Da Rocha, D. R., Da Silva, F. D. C., Moreirinha, C., Almeida, A., Ferreira, V. F., & Cavaleiro, J. A. S. (2017). Efficient Catalytic Oxidation of 3-Arylthio- and 3-Cyclohexylthio-lapachone Derivatives to New Sulfonyl Derivatives and Evaluation of Their Antibacterial Activities. Molecules, 22(2), 302. https://doi.org/10.3390/molecules22020302