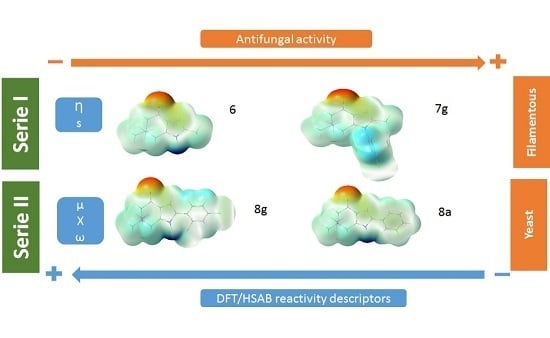
4.1. Global Reactivity Parameters
Table 2 shows the values of the calculated global chemical reactivity parameters for the 15 indol-4-ones compounds. The chemical reactivity values vary with the molecular structure and the substituent. According to the structural homology, the analyzed compounds were divided into two series: series I that includes compounds
6 and
7a–
g (N-1 substitution with phenyl moieties) and series II that includes compounds
8a to
8g (C-2 substitution with phenyl moieties).
Table 2 shows that for series I compound
6 has the highest hardness value (4.18 eV) and
7g has the lowest hardness value (3.80 eV); the difference is 0.38 eV. In contrast, for series II the highest hardness value (3.84 eV) corresponds to compound
8c and the lowest value (3.73 eV) to
8f and the difference is 0.11 eV. According to the maximum hardness principle, compounds
7g and
8f (
8g and
8d also) are more reactive than
6 and
8c, respectively. The electronegativity equalization principle assures in the course of a chemical reaction energetic stabilization through equalization of middle HOMO-LUMO levels among ligand and receptor active molecular structures [
38].
Table 2 reflects that compounds
7g in series I and
8g in series II present the highest electronegativity values (3.90 eV and 3.87 eV, respectively). The electrophilicity index
ω value for the same compounds (
7g 2.00 eV and
8g 2.01 eV), reflects the ability of
7g and
8g to behave as the stronger electrophiles on each series. The relative change between the maximum and minimum values of
ω in the Series I of
Table 2 (ω
max − ω
min/ω
max) = 0.21 is larger than the corresponding change of 0.17 for series II. This indicates that the capacity of series I to accept electrons (electrophilic character) is more sensitive to the specific substituent than series II.
Simple linear regression of the minimum inhibitory concentration (MIC) vs. global reactivity parameters for both series was obtained (
Table 3 and
Table 4). The Pearson coefficient was positive and the relationships were directly proportional: when the antifungal activity decreased, the global reactivity values increased. Then, when the global reactivity of those 15 indol-4-ones decreases, the higher antifungal activity is obtained. The best statistically significant relationships (the Pearson coefficient
p < 0.05) between both variables were obtained for yeast in series I: global hardness for
C. glabrata 48 h (r
η = 0.98),
C. krusei 24 h (r
η = 0.95),
C. tropicalis 24 h (r
η = 0.95),
C. guilliermondii 24 (r
η = 0.96) and 48 h (r
η = 0.94), and fungi:
A. fumigatus 72 h (r
η = 0.79) (
Table 3. This means a strong linear relationship between hardness and biological activity (96%, r
2 values until 0.96), with only 4% of variance of activity left to explain after taking into account the hardness in a linear way. For series II, global electronegativity and global electrophilicity index had a higher Pearson coefficient for
C. albicans 48 h and
C. glabrata 24 h (r
χ,ω = 0.98) and
C. tropicalis 48 h (r
χ = 0.82 and r
ω = 0.80) (
Table 4). This shows the same tendency as series I, with electronegativity and electrophilicity.
The relationship was strong for almost all cases, except for C. parapsilosis where the relationship did not have statistical significance.
Pearson coefficient in simple linear regression for series I had the following hierarchy from higher to lower values:
η >
ω >
χ while
χ =
ω >
η for series II. This could be related to results obtained by Putz et al. [
24], where they report values of monolinear correlation of activity of uracil derivatives (anti-HIV action) vs. chemical reactivity indices, and the tendency shown was
η >
ω >
χ, which is not the tendency one may expect obeying the established hierarchy for chemical binding scenario given by Putz [
39], according which a chemical reaction/interaction is triggered by the electronegativity difference, followed by chemical hardness and electrophilicity:
χ >
η >
ω, due to chemical–biological interactions. The higher Pearson coefficient presented by Putz is 0.67 for hardness, lower than the calculated value of the same parameter, 0.98. Although a different pharmacological activity is evaluated, it is possible to see the relation that can exist with these electronic properties of systems.
Stachowicz et al. evaluated thioamides derivatives and their activity against
C. albicans and correlated their activity vs. hardness, softness, and electrophilicity, with r values around 0.72 to 0.93 [
40]. These results coincide with those obtained by us with r values around 0.73 to 0.98; the chemical structure for thioamides are similar to indol-4-ones, =only in the presence of
N-heterocyclic system of five members, and this similarity could be responsible for similar correlations between biological activities and chemical reactivity parameters.
Different biological activities have been correlated with chemical reactivity parameters: hardness, softness, chemical potential, electronegativity, electrophilicity, and other electronic parameters looking for any relationship between electronic parameters and biological activity. Examples of studies with different parameters are: for testosterone derivatives r
ω = 0.42–0.94 [
10,
11]; carbonic anhydrase inhibitory r
χ,µ,S,εLUMO = 0.92 [
12]; anti HIV-1 integrase r
LogP,χ = 0.93 [
22]; anti-HIV activity with uracil derivatives r
χ = 0.24, r
η = 0.65, r
ω = 0.65, r
ω,χ = 0.69, and r
ω,η = 0.68, [
24]; etc. Although our analysis of antifungal activity does not match with those described above, the obtained values of r are better.
Multiple lineal regression for global reactivity indexes indicated that both hardness and softness are significant variables for series I (see
Table 5). The relationship was strong for
C. guilliermondii 24 h (r = 0.99) and 48 h (r = 0.99). Hardness and electrophilicity as well as hardness and chemical potential had strong relationship for fungi, and are indicated specifically for
A. fumigatus 48 h (r
η,s = 0.91) and 72 h (r
η,µ = 0.91). For series II, there is no statistically significance (
p > 0.05) linking two or more descriptors.
4.2. Local and Fragment Reactivity Parameters
The local Fukui function is related with the frontier controlled soft–soft interactions.
Figure 2 shows the isosurface plot of the Fukui function for an electrophilic attack
, and the positive values are shown in purple. For series I, the carbon atoms neighboring the nitrogen atom of the pyrrole ring are susceptible to be attacked by a soft electrophile followed by the oxygen atom of the carbonyl group and the vinylic carbon atoms of the pirrolic ring. For compounds in series II, the Fukui function shows the same reactive sites than series I. In addition, the carbon atom in the
para-position of the phenyl ring is susceptible for electrophilic attack.
Figure 3 shows the Fukui function for nucleophilic attack
, and the regions in purple color are positive values and show the most favorable sites for the attack of a soft nucleophile. For series I, the carbonyl and phenyl carbon atoms are prone to nucleophilic attack. For series II, these regions are the carbonyl group and carbon atoms from vinyl and phenyl ring.
The local Fukui function is localized within the carbonyl, pyrrole and phenyl moieties. In order to understand which molecular fragments are responsible for antifungal activity, the softness and electrophilicity were calculated for different fragments of compounds
6,
7a–
g and
8a–
g.
Table 6 shows the ID of the analyzed fragment, microorganisms, experimental time of testing, fragment chemical reactivity parameter, statistical correlation coefficient (r) for MIC and softness and electrophilicity fragments, and the atoms (marked in orange) considered in the fragment for series I and II. The basic fragment ID a (g and i) is related with the oxygen atom, fragment ID b (f and h) includes the carbon atom to gets the carbonyl group, fragment ID c includes carbon and nitrogen atoms from pyrrole ring and
ipso- and
ortho-carbon atoms of the phenyl ring, fragment ID d includes
meta and
para-carbon atoms of the phenyl ring, and so on. For
Aspergillus niger in series I, high correlation values were obtained for
sk− [fragment a = g (oxygen atom, r = 0.90, 48 h) and fragment b = f (carbonyl group, r = 0.93, 48 h)] and
sk+ [fragment c (r = 0.98, 72 h) and fragment d (r = 0.95, 48 h)]. As we can observe for
Aspergillus niger, the addition of the pyrrole and phenyl fragments to the carbonyl group increases the correlation coefficient for series I (the time of testing more representative for this species was 72 h). For
A. fumigatus, linear regressions for
sk+ (or
sk0) includes the carbonyl group r = 0.83 (or r = 0.82) and the oxygen atom r = 0.81 (or r = 0.80). The more significant time of testing was 48 h.
For series II, C. albicans has the higher correlation values for ωk−; the addition of carbon atom to oxygen atom to obtain the carbonyl group keeps the correlation for ωk− (see fragments m, r = 0.92 and n, r = 0.92). Fragments that include the nitrogen atom of the pyrrole ring do not increase the correlation value when increasing the number of carbon atoms of the phenyl ring (j, k and l, r = 0.75). The addition of the pyrrole ring, ipso carbon atom, and the meta-C atom from the phenyl ring, improves the correlation (q, r = 0.86). For other species, linear regressions with higher r were found when oxygen was included in ωk0: C. krusei 48 h (r = 0.78), C. tropicalis 24 h (r = 0.78), C. guilliermondii 48 h (r = 0.76), and C. parapsilosis 24 h (r = 0.78).
Additionally, the Parr functions [
41,
42] were calculated for electrophilic
P−(r) and nucleophilic
P+(r) attacks. They had similar tendency than Fukui functions (See
Tables S1 and S2).
4.3. MEP and Dipole Moment
Figure 4 shows the molecular electrostatic potential (MEP) for compounds
6,
7a–
g and
8a–
g. The MEP is a useful descriptor for understanding which sites in the molecules have affinity to a proton (charge controlled hard-hard interactions [
43]) and the relative polarity of the molecule [
44,
45,
46,
47,
48,
49,
50,
51,
52]. Regions in red color indicate higher negative charge, higher electron density, and higher affinity to a proton. Regions in blue color indicate more positive charge, a low electron density and a low affinity to a proton. For series I and II, the red region is located near the oxygen atom from carbonyl group, and the blue region is located near the nitrogen atom for both series, and the phenyl group for series I. In general, compounds in series I show a higher dipolar moment than compounds of series II as we can observe in
Table 7. The dipole moment was calculated for all of the 15 indol-4-ones at the B3LYP/6-311+G (d, p) level of theory. The dipole moment follows the trend:
7b >
7e >
7a >
8b >
6 >
7d =
8a >
7g >
8f >
8c >
7c >
7f >
8e >
8g >
8d. For series I, compound
7b shows the highest value (7.26 D), and, for series II, compound
8b has the highest value (6.69 D). Both compounds present a 2-fluor substitution in the aromatic ring. This can suggest that there is a correlation between the relative polarity of the compounds
6,
7a–
g and
8a–
g and the kind of interactions that these compounds can have with the active site of the receptor to antifungal activity.