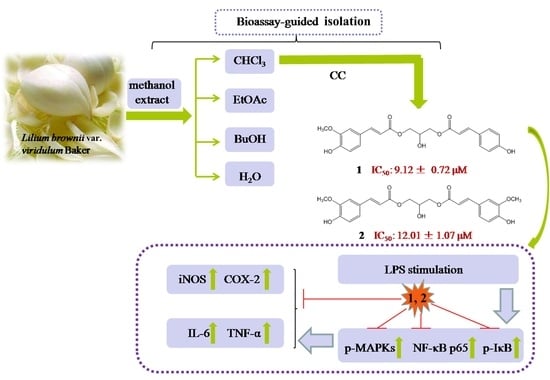
Bioassay-Guided Isolation of Anti-Inflammatory Components from the Bulbs of Lilium brownii var. viridulum and Identifying the Underlying Mechanism through Acting on the NF-κB/MAPKs Pathway
Abstract
:1. Introduction
2. Results
2.1. Phytochemical and Bioactive Screening of the Crude Extracts and Fractions of LB
2.2. Identification of Active Compounds and Evaluation of Their Cytotoxicities in RAW264.7 Macrophages
2.3. Anti-Inflammatory Activity Study of Compounds 1 and 2
2.4. Inhibitory Effects of Compounds 1 and 2 on TNF-α, IL-6 and IL-1β Expressions
2.5. Inhibition of MAPKs and NF-κB Signaling Pathways Accounts for the Anti-Inflammatory Effect of Compounds 1 and 2
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Preparation of the Extract, Bioassay-Guided Fractionation and Compounds Isolation
4.4. Cell Culture
4.5. Preparation of Samples
4.6. Measurement of Cell Viability
4.7. Nitrite Assay
4.8. Measurement of Pro-Inflammatory Cytokines
4.9. Total RNA Extraction and qRT-PCR
4.10. Preparation of Total and Nuclear Protein
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chau, C.F.; Wu, S.H. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci. Technol. 2006, 17, 313–323. [Google Scholar] [CrossRef]
- Munafo, J.P.; Gianfagna, T.J., Jr. Quantitative Analysis of Phenylpropanoid Glycerol Glucosides in Different Organs of Easter Lily (Lilium longiflorum Thunb.). J. Agric. Food Chem. 2015, 63, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Munafo, J.P.; Ramanathan, A.; Jimenez, L.S.; Gianfagna, T.J. Isolation and structural determination of steroidal glycosides from the bulbs of easter lily (Lilium longiflorum Thunb.). J. Agric. Food Chem. 2010, 58, 8806–8813. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yun, N.; Jang, Y.P.; Kim, J. Lilium lancifolium Thunb. extract attenuates pulmonary inflammation and air space enlargement in a cigarette smoke-exposed mouse model. J. Ethnopharmacol. 2013, 149, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.K.; Lee, M.Y.; Yuk, J.E.; Oh, S.R.; Chin, Y.W.; Lee, H.K.; Ahn, K.S. Anti-inflammatory effects of methanol extracts of the root of Lilium lancifolium on LPS-stimulated Raw264.7 cells. J. Ethnopharmacol. 2010, 130, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ariel, A.; Serhan, C.N. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007, 28, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Montgomery, K.E. Macrophage-mediated lymphangiogenesis: The emerging role of macrophages as lymphatic endothelial progenitors. Cancers 2012, 4, 618–657. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226, 229; discussion 230–232. [Google Scholar] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Becker, S.; Mundandhara, S.; Devlin, R.B.; Madden, M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol. Appl. Pharmacol. 2005, 207, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Jaffrey, S.R. Pharmacologic manipulation of nitric oxide signaling: Targeting NOS dimerization and protein-protein interactions. Curr. Top. Med. Chem. 2007, 7, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.U.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.F. Macrophages: Important physiologic and pathologic sources of polypeptide growth factors. Am. J. Respir. Cell Mol. Biol. 1990, 2, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Wun, Z.Y.; Lin, C.F.; Huang, W.C.; Huang, Y.L.; Xu, P.Y.; Chang, W.T.; Wu, S.J.; Liou, C.J. Anti-inflammatory effect of sophoraflavanone G isolated from Sophora flavescens in lipopolysaccharide-stimulated mouse macrophages. Food Chem. Toxicol. 2013, 62, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.S.; Bradshaw, H.B.; Chen, J.S.; Tan, B.; Walker, J.M. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFκB activity. Br. J. Pharmacol. 2008, 153, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Scherle, P.A.; Jones, E.A.; Favata, M.F.; Daulerio, A.J.; Covington, M.B.; Nurnberg, S.A.; Magolda, R.L.; Trzaskos, J.M. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J. Immunol. 1998, 161, 5681–5686. [Google Scholar] [PubMed]
- Chen, L.; Teng, H.; Fang, T.; Xiao, J. Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-κB in lipopolysaccharide-stimulated macrophages. Phytomedicine 2016, 23, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, H.; Sashida, Y.; Mimaki, Y. Phenolic glycerides from Lilium auratum. Phytochemistry 1987, 26, 844–845. [Google Scholar] [CrossRef]
- Luo, J.G.; Li, L.; Kong, L.Y. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem. 2012, 131, 1056–1062. [Google Scholar] [CrossRef]
- Delaporte, R.H.; Guzen, K.P.; Laverde, A.; dos Santos, A.R.; Sarragiotto, M.H. Phenylpropanoid glycerols from Tillandsia streptocarpa Baker (Bromeliaceae). Biochem. Syst. Ecol. 2006, 34, 599–602. [Google Scholar] [CrossRef]
- Hortelano, S.; Zeini, M.; Bosca, L. Nitric oxide and resolution of inflammation. Method Enzymol. 2002, 359, 459–465. [Google Scholar]
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lin, H.W.; Yang, D.J.; Chen, S.Y.; Tseng, J.K.; Chang, T.J.; Chang, Y.Y. Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-κB and activation of HO-1. Free Radic. Biol. Med. 2016, 95, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.L.; Qi, S.M.; Ling, L.F.; Lv, J.; Feng, Z.Y. Salidroside attenuates inflammatory response via suppressing JAK2-STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int. Immunopharmacol. 2016, 35, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.; Olsen, J.; Seidelin, J.B.; Nielsen, O.H. MAP kinases in inflammatory bowel disease. Clin. Chim. Acta 2011, 412, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Yoon, C.H.; Park, K.M.; Han, H.S.; Park, Y.K. Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-κB pathway. Food Chem. Toxicol. 2009, 47, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Torregrossa, A.C.; Parthasarathy, D.K.; Bryan, N.S. Natural Product Nitric Oxide Chemistry: New Activity of Old Medicines. Evid. Based Complement. Alternat. Med. 2012, 2012, 873210. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Q.; Hansson, G.K. Innate immunity, macrophage activation, and atherosclerosis. Immunol. Rev. 2007, 219, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, iNOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Rose-John, S.; Waetzig, G.H.; Scheller, J.; Grotzinger, J.; Seegert, D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin. Ther. Targets 2007, 11, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.L.; Sriskandan, S. Pro-inflammatory mechanisms in sepsis. Contrib. Microbiol. 2011, 17, 86–107. [Google Scholar] [PubMed]
- Whiteley, W.; Jackson, C.; Lewis, S.; Lowe, G.; Rumley, A.; Sandercock, P.; Wardlaw, J.; Dennis, M.; Sudlow, C. Inflammatory Markers and Poor Outcome after Stroke: A Prospective Cohort Study and Systematic Review of Interleukin-6. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Ruland, J. Return to homeostasis: Downregulation of NF-κB responses. Nat. Immunol. 2011, 12, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukocyte Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Heo, S.J.; Han, S.C.; Lee, H.J.; Kang, G.J.; Kang, H.K.; Hyun, J.W.; Koh, Y.S.; Yoo, E.S. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch. Pharm. Res. 2012, 35, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Ko, Y.J.; Kang, M.C.; Yang, H.M.; Roh, S.W.; Oda, T.; Jeon, Y.J.; Jung, W.K.; Heo, S.J.; Yoon, W.J.; et al. Anti-inflammatory effects of trans-1,3-diphenyl-2,3- epoxypropane-1-one mediated by suppression of inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 53, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Titheradge, M.A. The enzymatic measurement of nitrate and nitrite. Methods Mol. Biol. 1998, 100, 83–91. [Google Scholar] [PubMed]
- Cho, J.Y.; Baik, K.U.; Jung, J.H.; Park, M.H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur. J. Pharmacol. 2000, 398, 399–407. [Google Scholar] [CrossRef]
- Guo, C.; Yang, L.; Luo, J.; Zhang, C.; Xia, Y.; Ma, T.; Kong, L. Sophoraflavanone G from Sophora alopecuroides inhibits lipopolysaccharide-induced inflammation in RAW264.7 cells by targeting PI3K/Akt, JAK/STAT and Nrf2/HO-1 pathways. Int. Immunopharmacol. 2016, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Fan, B.Y.; Zhang, C.; Zhao, H.J.; Han, C.; Gao, C.Y.; Luo, J.G.; Kong, L.Y. Metabonomics applied in exploring the antitumour mechanism of physapubenolide on hepatocellular carcinoma cells by targeting glycolysis through the Akt-p53 pathway. Sci. Rep. 2016, 6, 29926. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1-O-feruloyl-2-O-p-coumaroylglycerol (1) and 1,3-O-diferuloylglycerol (2) are available from the authors. |







| Extracts/Fractions | IC50 (μg/mL) | Fractions | IC50 (μg/mL) a |
|---|---|---|---|
| LBM | 71.52 ± 4.52 * | Fr. A5 | 34.27 ± 1.34 |
| Fr. A | 32.70 ± 1.56 Δ | Fr. A3-1 | >50 |
| Fr. B | 64.35 ± 1.23 * | Fr. A3-2 | 18.30 ± 3.55 |
| Fr. C | 40.70 ± 0.92 Δ | Fr. A3-3 | 9.32 ± 1.24 |
| Fr. D | 163.11 ± 2.31 *,Δ | Fr. A3-4 | 26.91 ± 1.46 |
| Fr. A1 | >50 * | Fr. A3-5 | >50 |
| Fr. A2 | 37.58 ± 1.25 | Fr. A3-6 | 27.20 ± 2.72 |
| Fr. A3 | 11.49 ± 0.69 * | Fr. A3-7 | >50 |
| Fr. A4 | >50 * | l-NMMA b | 12.42 ± 2.50 |
| Compounds | Concentration (μM) | Mean % Viability | Mean % TNF-α Inhibition | Mean % IL-1β Inhibition | Mean % IL-6 Inhibition |
|---|---|---|---|---|---|
| 10 | 96.9 ± 2.6 | 22.6 ± 1.6 | 28.6 ± 1.9 | 23.8 ± 2.1 | |
| 1 | 20 | 94.8 ± 4.3 | 28.9 ± 2.7 | 45.3 ± 3.1 | 54.4 ± 2.9 |
| 30 | 95.0 ± 3.7 | 51.3 ± 4.9 | 55.9 ± 3.9 | 78.6 ± 5.0 | |
| 10 | 97.4 ± 2.8 | 18.7 ± 1.3 | 12.5 ± 0.8 | 18.1 ± 1.2 | |
| 2 | 20 | 95.2 ± 5.6 | 28.2 ± 3.1 | 23.6 ± 2.5 | 37.0 ± 3.2 |
| 30 | 96.0 ± 3.8 | 51.1 ± 4.7 | 43.3 ± 2.6 | 85.4 ± 5.3 | |
| 10 | 95.8 ± 2.9 | 58.3 ± 4.2 | 62.1 ± 4.3 | 58.7 ± 2.3 | |
| Dexamethasone | 20 | 101.2 ± 3.6 | 72.4 ± 3.2 | 69.7 ± 5.8 | 82.9 ± 4.7 |
| 30 | 97.2 ± 5.8 | 86.4 ± 6.2 | 80.4 ± 7.3 | 91.5 ± 7.9 |
| Gene | Sequence (5′ to 3′) |
|---|---|
| iNOS | Fw:GAATCTTGGAGCGAGTTGTGGA |
| Rv:GTGAGGGCTTGGCTGAGTGAG | |
| COX-2 | Fw:CTGGTGCCTGGTCTGATGATGT |
| Rv:AGTCTGCTGGTTTGGAATAGTTGCT | |
| TNF-α | Fw:CTTGTTGCCTCCTCTTTTGCTTA |
| Rv:CTTTATTTCTCTCAATGACCCGTAG | |
| IL-1β | Fw:TGTGTTTTCCTCCTTGCCTCTGAT |
| Rv:TGCTGCCTAATGTCCCCTTGAAT | |
| IL-6 | Fw:AAGGAGTGGCTAAGGACCAAGAC |
| Rv:AGTGAGGAATGTCCACAAACTGATA | |
| β-actin | Fw:ACCACACCTTCTACAATGAG |
| Rv:ACGACCAGAGGCATACAG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Wang, Z.; Zhang, Y.-M.; Luo, J.-G.; Kong, L.-Y. Bioassay-Guided Isolation of Anti-Inflammatory Components from the Bulbs of Lilium brownii var. viridulum and Identifying the Underlying Mechanism through Acting on the NF-κB/MAPKs Pathway. Molecules 2017, 22, 506. https://doi.org/10.3390/molecules22040506
Ma T, Wang Z, Zhang Y-M, Luo J-G, Kong L-Y. Bioassay-Guided Isolation of Anti-Inflammatory Components from the Bulbs of Lilium brownii var. viridulum and Identifying the Underlying Mechanism through Acting on the NF-κB/MAPKs Pathway. Molecules. 2017; 22(4):506. https://doi.org/10.3390/molecules22040506
Chicago/Turabian StyleMa, Ting, Zhen Wang, Yang-Mei Zhang, Jian-Guang Luo, and Ling-Yi Kong. 2017. "Bioassay-Guided Isolation of Anti-Inflammatory Components from the Bulbs of Lilium brownii var. viridulum and Identifying the Underlying Mechanism through Acting on the NF-κB/MAPKs Pathway" Molecules 22, no. 4: 506. https://doi.org/10.3390/molecules22040506
APA StyleMa, T., Wang, Z., Zhang, Y. -M., Luo, J. -G., & Kong, L. -Y. (2017). Bioassay-Guided Isolation of Anti-Inflammatory Components from the Bulbs of Lilium brownii var. viridulum and Identifying the Underlying Mechanism through Acting on the NF-κB/MAPKs Pathway. Molecules, 22(4), 506. https://doi.org/10.3390/molecules22040506