Synthesis and Antitumor Activity of New Group 3 Metallocene Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
- (i)
- Cyclopentadiene was obtained by a retro Diels–Alder of dicyclopentadiene at high temperature (180 °C).
- (ii)
- The synthesis of fulvene pro-ligands by reaction of cyclopentadiene with suitable aromatic aldehyde.
- (iii)
- Lithium salts of ligands were synthesized by reacting the appropriate fulvene with LiEt3BH (super-hydride), in dry diethyl ether. The reaction is a nucleophilic addition of a hydride to the double bond 5–6 of fulvene. We used a slight excess with respect to the stoichiometric amount of super-hydride. The choice of the Li(Et)3BH as reducing agent derived from its high selectivity. In fact, the double bond 5–6 of fulvene had a high polarity due to the inductive effect of the methoxy group bonded to benzene. By this increase of the polarity, the reducing agent attacked the double bond 5–6 and not the dienic component of fulvene [28,29,30].
- (iv)
- Synthesis of group 3 and lanthanide complexes were carried out in THF at −78 °C, by the reaction of a chosen lithium salt of ligands with the suitable stoichiometric amount of metal(III) halide, in order to obtain half- and metallocene derivatives and, consequently, to evaluate the influence of these lipophilic groups on the biological activity of the compounds.
Hydrolysis Stability
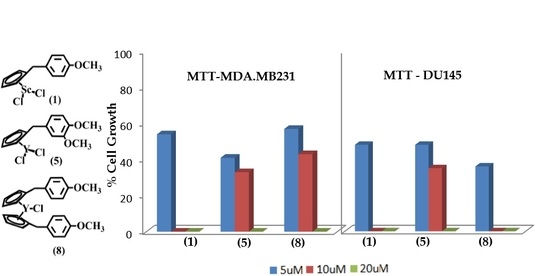
2.2. Biological Activity
2.3. Evaluation of the Drug-Membrane Interaction
3. Materials and Methods
3.1. General Procedure
3.2. Solvents
3.3. Reagents
3.4. Characterization Techniques
3.5. Cell Lines
3.6. Biological Assay Kit
3.7. Evaluation of the Drug-Membrane Interaction
3.7.1. Liposomes Preparation
3.7.2. Determination of the Partition Coefficient (Kp)
4. Synthesis of Ligands
4.1. Synthesis of Group 3 Metal Cyclopentadienyl Complexes
4.1.1. General Procedure
4.1.2. Yields and Spectral Data of Scandium Complexes
4.1.3. Yields and Spectral Data of Yttrium Complexes
4.1.4. Yields and Spectral Data of Neodymium Complexes
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rosenberg, B.; Van, C.L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis product from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum neurotoxicity pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharm. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.; Frasci, G.; Chirico, A.; Esposito, E.; Siani, C.; Saturnino, C.; Arra, C.; Ciliberto, G.; Giordano, A.; D'Aiuto, M. Triple negative breast cancer: looking for the missing link between biology and treatments. Oncotarget 2015, 6, 26560–26574. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Kasai, H.; Takeda, Y.; Maekawa, R.; Sugita, K.; Yoshioka, T. Synergy of combination of nedaplatin with etoposide in murine and human lung carcinoma. Anticancer Res. 1998, 18, 247–252. [Google Scholar] [PubMed]
- Misset, J.L. Oxaplatin in pratice. Br. J. Cancer 1998, 77, 4–7. [Google Scholar] [CrossRef]
- Kruh, G.D. Lustrous insights into cisplatin accumulation: Copper transporters. Clin. Cancer Res. 2003, 9, 5807–5809. [Google Scholar]
- Reedijk, J. Improved understanding in platinum antitumor chemistry. Chem. Commun. 1996, 7, 801–806. [Google Scholar] [CrossRef]
- Hambley, T.W. The influence of structure on the activity and toxicity of Pt anticancer drugs. Coord. Chem. Rev. 1997, 166, 181–223. [Google Scholar] [CrossRef]
- Augeà, P.; Kozelka, J. Transformations and recognition of platinum-DNA adducts: Recent developments. Transit. Met. Chem. 1997, 22, 91–96. [Google Scholar] [CrossRef]
- Saturnino, C.; Buonerba, M.; Boatto, G.; Pascale, M.; Moltedo, O.; de Napoli, L.; Montesarchio, D.; Lancelot, J.C.; de Caprariis, P. Synthesis and preliminary biological evaluation of a new pyridocarbazole derivative covalently linked to a thymidine nucleoside as a potential targeted antitumoral agent. Chem. Pharm. Bull. 2003, 51, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Zanella, A.; Gandin, V.; Porchia, M.; Refosco, F.; Tisato, F.; Sorrentino, F.; Scutari, G.; Rigobello, M.P. Cytotoxicity in human cancer cells and mitochondrial dysfunction induced by a series of new copper(I) complexes containing tris(2-cyanoethyl)phosphines. Investig. New Drugs 2011, 29, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cheng, Y.; Yang, X.; Li, R. Cell response to lanthanides and potential pharmacological actions of lanthanides. Metal Ions Biol. Syst. 2003, 40, 707–751. [Google Scholar]
- Evans, C.H. Interesting and useful biochemical properties of lanthanides. Trends Biochem. Sci. 1983, 8, 445–449. [Google Scholar] [CrossRef]
- Kostova, I.; Manolov, I.; Nicolova, I.; Konstantinov, S.; Karaivanova, M. New lanthanide complexes of 4-methyl-7-hydroxy coumarin and their pharmacological activity. Eur. J. Med. Chem. 2001, 36, 339–347. [Google Scholar] [CrossRef]
- Kostova, I.; Momekov, G.; Zaharieva, M.; Karaivanova, M. Cytotoxic activity of new lanthanum(III) complexes of bis-coumarins. Eur. J. Med. Chem. 2005, 40, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Thati, B.; Noble, A.; Creaven, B.S.; Walsh, M.; McCann, M.; Kavanagh, K.; Devereux, M.; Egan, D.A. A study of role of apoptopic cell death and cell cycle events mediatine the mechanism of action of 6-hydroxycoumarin-3-carboxylatosilver in human malignant hepatic cells. Cancer Lett. 2007, 250, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Trendafilova, N.; Momekov, G.J. Theoretical, spectral characterization and antineoplatic activity of new lantanide complexes. J. Trace Elem. Med. Biol. 2008, 22, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Miller, R.A. Texaphyrins: New drugs with diverse clinical applications in radiation and photodynamic therapy. Biochem. Pharmacol. 2000, 59, 733–739. [Google Scholar] [CrossRef]
- Mody, T.D.; Fu, L.; Sessler, J.L. Texaphyrins: Synthesis and development of novel class of therapeutic agents. Prog. Inorg. Chem. 2001, 49, 551–598. [Google Scholar]
- Biba, F.; Groessl, M.; Egger, A.; Roller, A.; Hartinger, C.G.; Keppler, B.K. New insights into the chemistry of the antineoplastic lanthanum complexes tris(1,10-phenanttroline)tris(thiocynato-kN)lanthanum(III)(KP772)and its interaction with biomolecules. Eur. J. Chem. 2009, 28, 4282–4287. [Google Scholar]
- Li, F.H.; Zhao, G.H.; Wu, H.X.; Lin, H.; Wu, X.X.; Zhu, S.R.; Lin, H.K. Synthesis, characterization and biological activity of lanthanum(III) complexes containing 2-methylene–1,10-phenanthroline units bridged by aliphatic diamines. J. Inorg. Biochem. 2006, 100, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Tan, M.X.; Liu, Y.C.; Peng, Y.; Wang, H.H.; Liu, H.G.; Liang, H. Synthesis, characterization and preliminary cytotoxicity evaluation of five Lanthanide(III)-Plumbagin complexes. J. Inorg. Biochem. 2011, 105, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Qiao, X.; Gao, C.Y.; Xu, G.J.; Wang, Z.L.; Tian, J.L.; Xu, J.Y.; Gu, W.; Liu, X.; Yan, S.P. Synthesis, DNA binding, photo-induced DNA cleavage and cell cytotoxity studies of a family of light rare earth complexes. J. Inorg. Bio. 2012, 109, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Gadadhar, S.; Goswami, T.K.; Karande, A.A.; Chakravarty, A.R. Photoactivated DNA cleavage and anticancer activity of pyrenyl-terpyridine lanthanide complexes. Eur. J. Med. Chem. 2012, 50, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xu, D.; Cheng, N.; Zhou, X.; Chen, X.; Xu, Y.; He, Q. Synthesis, characterization, and biological activity of some lanthanide ternary complexes. J. Coord. Chem. 2011, 64, 2342–2352. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Paolucci, G.; Fregona, D.; Dalla Via, L.; Enrichi, F. Group 3 and lanthanide triflate-complexes with [N,N,O]-donor ligands: Synthesis, characterization, and cytotoxic activity. J. Coord. Chem. 2012, 65, 3903–3916. [Google Scholar] [CrossRef]
- Saturnino, C.; Napoli, M.; Paolucci, G.; Bortoluzzi, M.; Popolo, A.; Pinto, A.; Longo, P. Synthesis and cytotoxic activities of group 3 metal complexes having monoanionic tridentate ligands. Eur. J. Med. Chem. 2010, 45, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Napoli, M.; Saturnino, C.; Sirignano, E.; Popolo, A.; Pinto, A.; Longo, P. Synthesis, characterization and cytotoxicity studies of methoxy alkyl substituted metallocenes. Eur. J. Med. Chem. 2011, 46, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Sirignano, E.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Pisano, A.; Lappano, R.; Maggiolini, M.; Longo, P. New titanocene derivatives with high antiproliferative activity against breast cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Saturnino, C.; Iacopetta, D.; Mazzotta, R.; Caruso, A.; Plutino, M.R.; Mariconda, A.; Ramunno, A.; Sinicropi, M.S.; Pezzi, V.; Longo, P. Inhibition of human topoisomerase I and II and anti-proliferative effects on MCF-7 cells by new titanocene complexes. Bioorg. Med. Chem. 2015, 23, 7302–7312. [Google Scholar] [CrossRef] [PubMed]
- Claffey, J.; Hogan, M.; Müller-Bunz, H.; Pampillón, C.; Tacke, M. Oxali-titanocene Y: A potent anticancer drug. Chem. Med. Chem. 2008, 3, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Claffey, J.; Gleeson, B.; Hogan, M.; Müller-Bunz, H.; Wallis, D.; Tacke, M. Fluorinated derivatives of titanocene Y: Synthesis and cytotoxicity studies. Eur. J. Inorg. Chem. 2008, 26, 4074–4082. [Google Scholar] [CrossRef]
- Sweeney, N.; Gallagher, W.M.; Müller-Bunz, H.; Pampillón, C.; Strohfeldt, K.; Tacke, M. Heteroaryl substituted titanocenes as potential anti-cancer drugs. J. Inorg. Biochem. 2006, 100, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.; Moutinho, C.; Lobão, P. Liposomes as a model for the biological membrane: Studies on daunorubicin bilayer interaction. J. Membr. Biol. 2012, 245, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Paesano, N.; Marzocco, S.; Vicidomini, C.; Saturnino, C.; Autore, G.; De Martino, G.; Sbardella, G. Synthesis and biological evaluation of 3-benzyl-1-methyl- and 1-methyl-3-phenyl-isothioureas as potential inhibitors of iNOS. Bioorg. Med. Chem. Lett. 2005, 15, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Sirignano, E.; Saturnino, C.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Pisano, A.; Lappano, R.; Maggiolini, M.; Longo, P. Synthesis, characterization and cytotoxic activity on breast cancer cells of new half-titanocene derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 3458–3462. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Sinicropi, M.S.; Lancelot, J.C.; El-Kashef, H.; Saturnino, C.; Aubert, G.; Belladonne, C.; Lesnard, A.; Cresteil, T.; Dallemagne, P.; Rault, S. Synthesis and evaluation of cytotoxix activities of new guanidines derived from carbazole. Bioorg. Med. Chem. Lett. 2014, 24, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Napoli, M.; Saturnino, C.; Cianciulli, E.; Varcamonti, M.; Zanfardino, A.; Tommonaro, G.; Longo, P. Silver(I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Org. Chem. 2013, 60, 112–119. [Google Scholar] [CrossRef]
- Sirignano, E.; Pisano, A.; Caruso, A.; Saturnino, C.; Sinicropi, M.S.; Lappano, R.; Botta, A.; Iacopetta, D.; Maggiolini, M.; Longo, P. Different 6-Aryl-Fulvenes Exert Anti-proliferative effects on Cancer Cells. Anti-Cancer Agents Med. Chem. 2015, 15, 468–474. [Google Scholar] [CrossRef]
- Theile, D. Under-Reported aspects of Platinum Drug Pharmacology. Molecules 2017, 22, 382. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Compound | R | M | Name |
|---|---|---|---|
| 1 | H | Sc | [(p-methoxybenzyl)cyclopentadienyl]scandium dichloride |
| 2 | H | Y | [(p-methoxybenzyl)cyclopentadienyl]yttrium dichloride |
| 3 | H | Nd | [(p-methoxybenzyl)cyclopentadienyl]neodymium dichloride |
| 4 | -OCH3 | Sc | [(3,4-dimethoxybenzyl)cyclopentadienyl]scandium dichloride |
| 5 | -OCH3 | Y | [(3,4-dimethoxybenzyl)cyclopentadienyl]yttrium dichloride |
| 6 | -OCH3 | Nd | [(3,4-dimethoxybenzyl)cyclopentadienyl]neodymium dichloride |
| 7 | H | Sc | bis-[(p-methoxybenzyl)cyclopentadienyl]scandium chloride |
| 8 | H | Y | bis-[(p-methoxybenzyl)cyclopentadienyl]yttrium chloride |
| 9 | H | Nd | bis-[(p-methoxybenzyl)cyclopentadienyl]neodymium chloride |
| 10 | -OCH3 | Sc | bis-[(3,4-dimethoxybenzyl)cyclopentadienyl]scandium chloride |
| 11 | -OCH3 | Y | bis-[(3,4-dimethoxybenzyl)cyclopentadienyl]yttrium chloride |
| 12 | -OCH3 | Nd | bis-[(3,4-dimethoxybenzyl)cyclopentadienyl]neodymium chloride |
| MDA.MB231 | DU145 | ||||
|---|---|---|---|---|---|
| Scandium | Yttrium | Neodymium | Scandium | Yttrium | Neodymium |
| Compounds IC50 (µM) | Compounds IC50 (µM) | Compounds IC50 (µM) | Compounds IC50 (µM) | Compounds IC50 (µM) | Compounds IC50 (µM) |
| 1 <10 | 5 <5 | 3 12 | 1 <10 | 5 <5 | 3 12 |
| 4 <20 | 8 <5 | 6 50 | 4 <20 | 8 <5 | 6 50 |
| 7 20 | 11 <50 | 7 <20 | 11 <50 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporale, A.; Palma, G.; Mariconda, A.; Del Vecchio, V.; Iacopetta, D.; Parisi, O.I.; Sinicropi, M.S.; Puoci, F.; Arra, C.; Longo, P.; et al. Synthesis and Antitumor Activity of New Group 3 Metallocene Complexes. Molecules 2017, 22, 526. https://doi.org/10.3390/molecules22040526
Caporale A, Palma G, Mariconda A, Del Vecchio V, Iacopetta D, Parisi OI, Sinicropi MS, Puoci F, Arra C, Longo P, et al. Synthesis and Antitumor Activity of New Group 3 Metallocene Complexes. Molecules. 2017; 22(4):526. https://doi.org/10.3390/molecules22040526
Chicago/Turabian StyleCaporale, Angelamaria, Giuseppe Palma, Annaluisa Mariconda, Vitale Del Vecchio, Domenico Iacopetta, Ortensia Ilaria Parisi, Maria Stefania Sinicropi, Francesco Puoci, Claudio Arra, Pasquale Longo, and et al. 2017. "Synthesis and Antitumor Activity of New Group 3 Metallocene Complexes" Molecules 22, no. 4: 526. https://doi.org/10.3390/molecules22040526
APA StyleCaporale, A., Palma, G., Mariconda, A., Del Vecchio, V., Iacopetta, D., Parisi, O. I., Sinicropi, M. S., Puoci, F., Arra, C., Longo, P., & Saturnino, C. (2017). Synthesis and Antitumor Activity of New Group 3 Metallocene Complexes. Molecules, 22(4), 526. https://doi.org/10.3390/molecules22040526