Synthesis and Antibacterial Evaluation of Novel 3-Substituted Ocotillol-Type Derivatives as Leads
Abstract
:1. Introduction
2. Results and Discussion
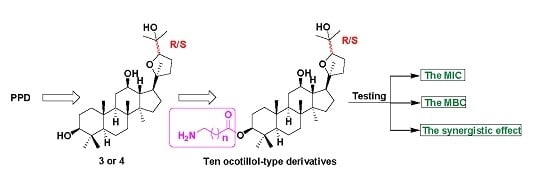
2.1. Synthesis of Ocotillol-Type Derivatives
2.2. Antibacterial Activity
2.3. Synergistic Antibacterial Activity
2.4. The Structure-Activity Relationships (SARs) of Ocotillol-Type Derivatives
3. Materials and Methods
3.1. Chemical Reagents and Instruments
3.2. General Procedure for the Synthesis of (20S,24S)-Epoxy-dammarane-3β,12β,25-triol (OS) and (20S,24R)–Epoxy-dammarane-3β,12β,25-triol (OR)
3.3. General Procedure for the Synthesis of Compounds 5a–e and 6a–e
3.4. Pharmacology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yap, P.S.X.; Lim, S.H.; Hu, C.P.; Yia, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Jassim, S.A.; Limoges, R.G. Natural solution to antibiotic resistance: Bacteriophages ‘The Living Drugs’. World J. Microbiol. Biotechnol. 2014, 30, 2153–2170. [Google Scholar] [CrossRef] [PubMed]
- Carrel, M.; Perencevich, E.N.; David, M.Z. USA300 Methicillin-Resistant Staphylococcus aureus, United States, 2000–2013. Emerg. Infect. Dis. 2015, 21, 1973. [Google Scholar] [CrossRef] [PubMed]
- Boyle-Vavra, S.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus: The role of Panton–Valentine leukocidin. Lab. Investig. 2007, 87, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Ochieng, D.J.; Levy, S.B. Probing the role of commensals in propagating antibiotic resistance should help preserve the efficacy of these critical drugs. Microbe Mag. 2009, 4, 231–238. [Google Scholar] [CrossRef]
- Loomba, P.S.; Taneja, J.; Mishra, B. Methicillin and vancomycin resistant S. aureus in hospitalized patients. J. Glob. Infect. Dis. 2010, 2, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, X.O.; Liu, X.F.; Wang, Z.J.; Gao, X.; Sun, N.N.; Huang, Y.; Liu, N. Research progress on MRSA and its resistance to vancomycin. Chin. J. Lab. Diagn. 2016, 10, 1785–1787. [Google Scholar]
- Bogusz, M.J.; Hassan, H.; Al-Enazi, E.; Ibrahim, Z.; Al-Tufail, M. Rapid determination of chloramphenicol and its glucuronide in food products by liquid chromatography-electrospray negative ionization tandem mass spectrometry. J. Chromatogr. B 2004, 807, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandan, K.; Swamy, M.G.; Prasad, S.; Mukherjee, R. A simple method of isolation of chloramphenicol in honey and its estimation by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.J.; Suh, M.W.; Ahn, S.C. Sustained Fos expression is observed in the developing brainstem auditory circuits of kanamycin-treated rats. Neurosci. Lett. 2011, 505, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Yin, Z.D.; Fan, G.R.; Li, D.; Huang, X. Time sequence of auditory nerve and spiral ganglion cell degeneration following chronic kanamycin-induced deafness in the guinea pig. Brain Res. 2010, 1331, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Price, K.R.; Johnson, I.T.; Fenwick, G.R.; Malinow, M.R. The chemistry and biological significance of saponins in foods and feedingstuffs. Crit. Rev. Food Sci. Nutr. 1987, 26, 27–135. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Saeed Saify, Z. Naturally occurring and synthetic agents as potential anti-inflammatory and immunomodulants. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 3–19. [Google Scholar] [CrossRef]
- Wolska, K.; Grudniak, A.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Open Life Sci. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- MR Patlolla, J.; V Rao, C. Triterpenoids for cancer prevention and treatment: current status and future prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef]
- Cassels, B.K.; Asencio, M. Anti-HIV activity of natural triterpenoids and hemisynthetic derivatives 2004–2009. Phytochem. Rev. 2011, 10, 545–564. [Google Scholar] [CrossRef]
- Warnhoff, E.W.; Halls, C.M.M. Desert Plant Constituents: II. Ocotillol: An Intermidiate in the Oxidation of Hydroxy Isooctenyl Side Chains. Can. J. Chem. 1965, 43, 3311–3321. [Google Scholar] [CrossRef]
- Lee, D.G.; Chang, Y.S.; Yoonkyung, P.; Hahm, K.S.; Woo, E.R. Antimicrobial effects of ocotillone isolated from stem bark of Ailanthus altisshima. J. Microbiol. Biotechnol. 2002, 12, 854–857. [Google Scholar]
- Bi, Y.; Tian, J.; Wang, L.; Zhao, F.; Zhang, J.; Wang, N.; Meng, Q. Synthesis, structural determination and protective effects on cultured anoxia/reoxygen injury myocardiocytes of ocotillol-type derivatives. J. Med. Plants Res. 2011, 5, 2424–2429. [Google Scholar]
- Bi, Y.; Ma, C.; Zhang, H.; Zhou, Z.; Yang, J.; Zhang, Z.; Xu, J. Novel 3-Substituted Ocotillol-Type Triterpenoid Derivatives as Antibacterial Candidates. Chem. Boil. Drug Des. 2014, 84, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Yang, X.; Zhang, T.; Liu, Z.; Zhang, X.; Lu, J.; Lewis, P.J. Design, synthesis, nitric oxide release and antibacterial evaluation of novel nitrated ocotillol-type derivatives. Eur. J. Med. Chem. 2015, 101, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, C.; Zhang, H.; Bi, Y.; Chen, X.; Tian, H.; Xu, J. Synthesis and biological evaluation of novel ocotillol-type triterpenoid derivatives as antibacterial agents. Eur. J. Med. Chem. 2013, 68, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.; Ip, M.; Gong, H.; Lui, S.L.; See, R.H.; Jolivalt, C.; Lau, C.B. Synergistic effects of diosmetin with erythromycin against ABC transporter over-expressed methicillin-resistant Staphylococcus aureus (MRSA) RN4220/pUL5054 and inhibition of MRSA pyruvate kinase. Phytomedicine 2013, 20, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ma, C.; Zhou, Z.; Zhang, T.T.; Zhang, H.Y.; Zhang, X.C.; Lu, J.; Meng, Q.G.; Lewis, P.J.; Xu, J.Y. Synthesis and Antibacterial Evaluation of Novel Hydrophilic Ocotillol-Type Triterpenoid Derivatives from 20(S)-Protopanaxadiol. Rec. Nat. Prod. 2015, 9, 356–368. [Google Scholar]
- Ma, C.; Yang, X.; Kandemir, H.; Mielczarek, M.; Johnston, E.B.; Griffith, R.; Lewis, P.J. Inhibitors of bacterial transcription initiation complex formation. ACS Chem. Biol. 2013, 8, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Y.; Liu, X.-X.; Zhang, H.-Y.; Yang, X.; Liu, Z.-Y.; Lu, J.; Lewis, P.J.; Wang, C.-Z.; Xu, J.-Y.; Meng, Q.-G.; et al. Synthesis and Antibacterial Evaluation of Novel 3-Substituted Ocotillol-Type Derivatives as Leads. Molecules 2017, 22, 590. https://doi.org/10.3390/molecules22040590
Bi Y, Liu X-X, Zhang H-Y, Yang X, Liu Z-Y, Lu J, Lewis PJ, Wang C-Z, Xu J-Y, Meng Q-G, et al. Synthesis and Antibacterial Evaluation of Novel 3-Substituted Ocotillol-Type Derivatives as Leads. Molecules. 2017; 22(4):590. https://doi.org/10.3390/molecules22040590
Chicago/Turabian StyleBi, Yi, Xian-Xuan Liu, Heng-Yuan Zhang, Xiao Yang, Ze-Yun Liu, Jing Lu, Peter John Lewis, Chong-Zhi Wang, Jin-Yi Xu, Qing-Guo Meng, and et al. 2017. "Synthesis and Antibacterial Evaluation of Novel 3-Substituted Ocotillol-Type Derivatives as Leads" Molecules 22, no. 4: 590. https://doi.org/10.3390/molecules22040590
APA StyleBi, Y., Liu, X.-X., Zhang, H.-Y., Yang, X., Liu, Z.-Y., Lu, J., Lewis, P. J., Wang, C.-Z., Xu, J.-Y., Meng, Q.-G., Ma, C., & Yuan, C.-S. (2017). Synthesis and Antibacterial Evaluation of Novel 3-Substituted Ocotillol-Type Derivatives as Leads. Molecules, 22(4), 590. https://doi.org/10.3390/molecules22040590