A Fluorescent Probe for Glycosaminoglycans Applied to the Detection of Dermatan Sulfate by a Mix-and-Read Assay
Abstract
:1. Introduction
2. Results and Discussion
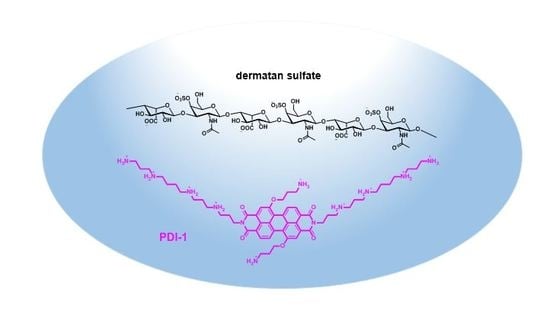
2.1. Synthesis and Properties of the Polyamine-Functionalized Perylene Diimide Probe PDI-1
2.2. Detection of Dermatan Sulfate in Aqueous Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.1.1. Heparin Red Kit
3.1.2. Dermatan Sulfate
3.1.3. Plasma
3.1.4. Other
3.2. Instrumentation
3.2.1. Analytics
3.2.2. Fluorescence Measurements
3.2.3. Microplates, Cuvettes, Pipettes
3.3. Synthesis
3.3.1. Synthesis of N,N’-Bis-(N1,N4,N9,N13-tetra-tert-butyloxycarbonyl-1-amino-4,9,13-triazahexadecyl)-1,7-dibromperylene-3,4:9,10-tetracarboxylic acid bisimide PDI-3
3.3.2. Synthesis of N,N’-Bis-(1-amino-4,9,13-triazahexadecyl)-1,7-di-(1-amino-3-hydroxypropyl)-perylene-3,4:9,10-tetracarboxydiimide PDI-1
3.4. Assays
Dermatan Sulfate Assay in Aqueous and Plasma Matrix
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bromfield, S.M.; Wilde, E.; Smith, D.K. Heparin sensing and binding—Taking supramolecular chemistry towards clinical applications. Chem. Soc. Rev. 2013, 42, 9184–9195. [Google Scholar] [CrossRef] [PubMed]
- Szelke, H.; Schübel, S.; Harenberg, J.; Krämer, R. A fluorescent probe for the quantification of heparin in clinical samples with minimal matrix interference. Chem. Commun. 2010, 46, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Warttinger, U.; Giese, C.; Harenberg, J.; Holmer, E.; Krämer, R. A fluorescent probe assay (Heparin Red) for direct detection of heparins in human plasma. Anal. Bioanal. Chem. 2016, 408, 8241–8251. [Google Scholar] [CrossRef] [PubMed]
- Warttinger, U.; Krämer, R. Quantification of heparin in complex matrices (including urine) using a mix-and-read fluorescence assay. arXiv, 2016; arXiv:1611.02482. [Google Scholar]
- Galli, M.; Magen, H.; Einsele, H.; Chatterjee, M.; Grasso, M.; Specchia, G.; Barbieri, P.; Paoletti, D.; Pace, S.; Sanderson, R.D.; et al. Roneparstat (SST0001), an Innovative Heparanase (HPSE) Inhibitor for Multiple Myeloma (MM) Therapy: First in Man Study. Blood 2015, 126, 3246. [Google Scholar]
- Szelke, H.; Schübel, S.; Harenberg, J.; Krämer, R. Interaction of heparin with cationic molecular probes: Probe charge is a major determinant of binding stoichiometry and affinity. Bioorg. Med. Chem. Lett. 2010, 20, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Warttinger, U.; Krämer, R. Instant determination of the potential biomarker heparan sulfate in human plasma by a mix-and-read fluorescence assay. arXiv, 2017; arXiv:1702.05288. [Google Scholar]
- Warttinger, U.; Giese, C.; Harenberg, J.; Krämer, R. Direct quantification of brown algae-derived fucoidans in human plasma by a fluorescent probe assay. arXiv, 2016; arXiv:1608.00108. [Google Scholar]
- Groß, N.; Arian, D.; Warttinger, U.; Krämer, R. Ultrasensitive quantification of dextran sulfate by a mix-and-read fluorescent probe assay. arXiv, 2017; arXiv:1703.08663. [Google Scholar]
- Trowbridge, J.M.; Gallo, R.L. Dermatan sulfate: New functions from an old glycosaminoglycan. Glycobiology 2002, 12, 117R–125R. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Marco, G.; Giangiacomo, T. Structural and Conformational Aspects of the Anticoagulant and Antithrombotic Activity of Heparin and Dermatan Sulfate. Curr. Pharm. Des. 2004, 10, 939–949. [Google Scholar]
- Mashima, R.; Sakai, E.; Tanaka, M.; Kosuga, M.; Okuyama, T. The levels of urinary glycosaminoglycans of patients with attenuated and severe type of mucopolysaccharidosis II determined by liquid chromatography-tandem mass spectrometry. Mol. Genet. Metab. Rep. 2016, 7, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Saivin, S.; Cambus, J.-P.; Thalamus, C.; Lau, G.; Boneu, B.; Houin, G.; Gianese, F. Pharmacokinetics and Pharmacodynamics of Intramuscular Dermatan Sulfate Revisited. Clin. Drug Investig. 2003, 23, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Coccheri, S.; Mannello, F. Development and use of sulodexide in vascular diseases: Implications for treatment. Drug Des. Dev. Ther. 2014, 8, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Lavoie, P.; Tomatsu, S.; Valayannopoulos, V.; Mitchell, J.J.; Raiman, J.; Beaudoin, M.; Maranda, B.; Clarke, J.T.R. UPLC-MS/MS detection of disaccharides derived from glycosaminoglycans as biomarkers of mucopolysaccharidoses. Anal. Chim. Acta 2016, 936, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Berutti, S.; Bagnis, C.; Soragna, G.; Gabella, P.; Fruttero, C.; Marangella, M. Dermatan sulfate: An alternative to unfractionated heparin for anticoagulation in hemodialysis patients. J. Nephrol. 2013, 26, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Geall, A.J.; Blagbrough, I.S. Homologation of Polyamines in the Rapid Synthesis of Lipospermine Conjugates and Related Lipoplexes. Tetrahedron 2000, 56, 2449–2460. [Google Scholar] [CrossRef]
- Boehm, A.; Arms, H.; Henning, G.; Blaschka, P. 1,7-Disubstituierte Perylen-3,4,9-10-tetracarbonsäuren, deren Dianhydride und Diimide. BASF AG Germany, Patent DE19547210A1, 19 June 1997. [Google Scholar]
- Volpi, N.; Maccari, F. Microdetermination of chondroitin sulfate in normal human plasma by fluorophore-assisted carbohydrate electrophoresis (FACE). Clin. Chim. Acta 2005, 356, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, E.; Lepedda, A.J.; Cigliano, A.; Pisanu, S.; Zinellu, A.; Carru, C.; Bacciu, P.P.; Piredda, F.; Guarino, A.; Spirito, R.; et al. Association between Human Plasma Chondroitin Sulfate Isomers and Carotid Atherosclerotic Plaques. Biochem. Res. Int. 2012, 2012, 281284. [Google Scholar] [CrossRef] [PubMed]
- Mix-and-Read Assays for Heparins. Available online: www.redprobes.com (accessed on 05 May 2017).
- Rappold, M. Perylendiimid-Basierte Fluoreszenzfarbstoffe: Synthese, Detektion von Glykosaminoglykanen und Photoinduzierte Zyklisierungsreaktionen. Ph.D. Thesis, Heidelberg University, Heidelberg, Germany, 2016. [Google Scholar]
- Guerrini, M.; Zhang, Z.; Shriver, Z.; Naggi, A.; Masuko, S.; Langer, R.; Casu, B.; Linhardt, R.J.; Torri, G.; Sasisekharan, R. Orthogonal analytical approaches to detect potential contaminants in heparin. Proc. Natl. Acad. Sci. USA 2009, 106, 16956–16961. [Google Scholar] [CrossRef] [PubMed]
- Carnachan, S.M.; Hinkley, S.F.R. Heparan Sulfate Identification and Characterisation: Method I. Heparan Sulfate Identification by NMR Analysis. Bio-Protocol 2017, 7, e2196. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Poeck, A. Ionische Fluoreszenzsonden zur Heparinbestimmung: Synthese und Anwendung. Ph.D. Thesis, Heidelberg University, Heidelberg, Germany, 2013. [Google Scholar]
Sample Availability: Samples of the compounds are not available |







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rappold, M.; Warttinger, U.; Krämer, R. A Fluorescent Probe for Glycosaminoglycans Applied to the Detection of Dermatan Sulfate by a Mix-and-Read Assay. Molecules 2017, 22, 768. https://doi.org/10.3390/molecules22050768
Rappold M, Warttinger U, Krämer R. A Fluorescent Probe for Glycosaminoglycans Applied to the Detection of Dermatan Sulfate by a Mix-and-Read Assay. Molecules. 2017; 22(5):768. https://doi.org/10.3390/molecules22050768
Chicago/Turabian StyleRappold, Melissa, Ulrich Warttinger, and Roland Krämer. 2017. "A Fluorescent Probe for Glycosaminoglycans Applied to the Detection of Dermatan Sulfate by a Mix-and-Read Assay" Molecules 22, no. 5: 768. https://doi.org/10.3390/molecules22050768
APA StyleRappold, M., Warttinger, U., & Krämer, R. (2017). A Fluorescent Probe for Glycosaminoglycans Applied to the Detection of Dermatan Sulfate by a Mix-and-Read Assay. Molecules, 22(5), 768. https://doi.org/10.3390/molecules22050768