Direct Analyses of Secondary Metabolites by Mass Spectrometry Imaging (MSI) from Sunflower (Helianthus annuus L.) Trichomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Verification of Target Compounds by Ultra-Performance Liquid Chromatography with Diode Array Dectection and Mass Spectrometry (UPLC-DAD-MS)
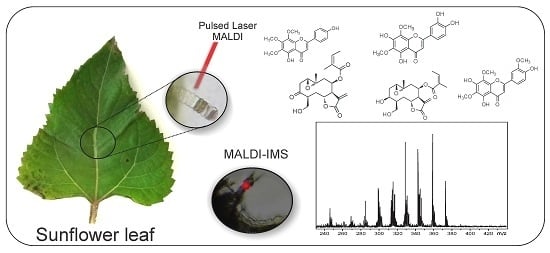
2.2. Optimization and MALDI/LDI-MSI
3. Materials and Methods
3.1. Plant Material
3.2. Ultra-Performance Liquid Chromatography with Diode Array Dectection and Mass Spectrometry (UPLC-DAD-MS) Analyses
3.3. MALDI-MS and LDI-MS of LGT Extracts and Standards
3.4. MALDI and LDI Imaging
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tissier, A. Glandular trichomes: What comes after expressed sequence tags? Plant J. 2012, 70, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Werker, E. Trichome diversity and development. Adv. Bot. Res. 2000, 31, 1–30. [Google Scholar]
- Zajaczkowska, U.; Kucharski, S.; Guzek, D. Are trichomes involved in the biomechanical systems of Cucurbita leaf petioles? Planta 2015, 242, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the Plant Trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.; Winters, J.N. Demonstration of the intercellular compartmentation of L-menthone metabolism in peppermint (Mentha piperita) leaves. Plant Physiol. 1982, 69, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.W.; Bass, W.T.; Houtz, R.L.; Wagner, G.J. Phylloplanins of tobacco are defensive proteins deployed on aerial surfaces by short glandular trichomes. Plant Cell 2005, 17, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Kroumova, A.B.; Wagner, G.G. Different elongation pathways in the biosynthesis of acyl groups of trichome exudate sugar esters from various solanaceous plants. Planta 2003, 216, 1013–1021. [Google Scholar] [PubMed]
- Gang, D.R.; Wang, J.; Dudareva, N.; Nam, K.H.; Simon, J.E.; Lewinsohn, E.; Pichersky, E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 2001, 125, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, A.-K.; Kwon, M.; Conrad, J.; Ro, D.-K.; Spring, O. Identification and characterization of two bisabolene synthases from linear glandular trichomes of sunflower (Helianthus annuus L., Asteraceae). Phytochemistry 2016, 124, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Göpfert, J.C.; MacNevin, G.; Ro, D.-K.; Spring, O. Identification, functional characterization and developmental regulation of sesquiterpene synthases from sunflower capitate glandular trichomes. BMC Plant Biol. 2009, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Göpfert, J.C.; Bülow, A.-K.; Spring, O. Identification and functional characterization of a new sunflower germacrene A synthase (HaGAS3). Nat. Prod. Commun. 2010, 5, 709–715. [Google Scholar] [PubMed]
- Nguyen, D.T.; Göpfert, J.C.; Ikezawa, N.; MacNevin, G.; Kathiresan, M.; Conrad, J.; Spring, O.; Ro, D.-K. Biochemical conservation and evolution of germacrene a oxidase in Asteraceae. J. Biol. Chem. 2010, 285, 16588–16598. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, N.; Göpfert, J.C.; Nguyen, D.T.; Kim, S.-U.; O’Maille, P.E.; Spring, O.; Ro, D.K. Lettuce costunolide synthase (CYP71BL2) and its homolog (CYP71BL1) from sunflower catalyze distinct regio- and stereoselective hydroxylations in sesquiterpene lactone metabolism. J. Biol. Chem. 2011, 286, 21601–21611. [Google Scholar] [CrossRef] [PubMed]
- Schwekendiek, A.; Spring, O.; Heyerick, A.; Pickel, B.; Pitsch, N.T.; Peschke, F.; De Keukeleire, D.; Weber, G. Constitutive expression of a grapevine stilbene synthase gene in transgenic hop (Humulus lupulus L.) yields high amounts resveratrol and its derivatives. J. Agric. Food Chem. 2007, 55, 7002–7009. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Last, R.L.; Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008, 54, 702–711. [Google Scholar]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef] [PubMed]
- Spring, O. Trichome microsampling of sesquiterpene lactones for the use of systematic studies. Recent Adv. Phytochem. 1991, 25, 319–345. [Google Scholar]
- Vogler, B.; Spring, O. Application of LC-NMR measurements in phytochemical studies. Recent Res. Dev. Phytochem. 2000, 4, 207–222. [Google Scholar]
- Misra, B.B.; Assmann, S.M.; Chen, S. Plant single-cell and single-cell-type metabolomics. Trends Plant Sci. 2014, 19, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Bjarnholt, N.; Li, B.; D’Alvise, J.; Janfelt, C. Mass spectrometry imaging of plant metabolites—Principles and possibilities. Nat. Prod. Rep. 2014, 31, 818–837. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Jones, A.D. Chemical imaging of trichome specialized metabolites using contact printing and laser desorption/ionization mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, D.; Shroff, R.; Knop, K.; Gottschaldt, M.; Crecelius, A.; Schneider, B.; Heckel, D.G.; Schubert, U.S.; Svatos, A. Matrix-free UV-laser desorption/ionization (LDI) mass spectrometric imaging at the single-cell level: Distribution of secondary metabolites of Arabidopsis thaliana and Hypericum species. Plant J. 2009, 60, 907–918. [Google Scholar]
- Lopes, A.A.; Pina, E.S.; Silva, D.B.; Pereira, A.M.S.; Silva, M.F.G.F.; Da Costa, F.B.; Lopes, N.P.; Pupo, M.T. A biosynthetic pathway of sesquiterpene lactones in Smallanthus sonchifolius and their localization in leaf tissues by MALDI imaging. Chem. Commun. 2013, 49, 9989–9991. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, A.-K.; Amrehn, E.; Bechtel, L.; Spring, O. Trichome differentiation on leaf primordia of Helianthus annuus (Asteraceae): Morphology, gene expression and metabolite profile. Planta 2015, 241, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Göpfert, J.C.; Heil, N.; Conrad, J.; Spring, O. Cytological development and sesquiterpene lactone secretion in capitate glandular trichomes of sunflower. Plant Biol. 2005, 7, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Amrehn, E.; Heller, A.; Spring, O. Capitate glandular trichomes of Helianthus annuus (Asteraceae): Ultrastructure and cytological development. Protoplasma 2014, 251, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Amrehn, E.; Aschenbrenner, A.-K.; Heller, A.; Spring, O. Localization of sesquiterpene lactone biosynthesis in cells of capitate glandular trichomes of Helianthus annuus (Asteraceae). Protoplasma 2016, 253, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Spring, O.; Benz, T.; Ilg, M. Sesquiterpene lactones of the capitate glandular trichomes of Helianthus annuus. Phytochemistry 1989, 28, 745–749. [Google Scholar] [CrossRef]
- Spring, O.; Pfannstiel, J.; Klaiber, I.; Conrad, J.; Beifuß, U.; Apel, L.; Aschenbrenner, A.-K.; Zipper, R. The nonvolatile metabolome of sunflower linear glandular trichomes. Phytochemistry 2015, 119, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Spring, O.; Rodon, U.; Macias, F.A. Sesquiterpenes from noncapitate glandular trichomes of Helianthus annuus. Phytochemistry 1992, 31, 1541–1544. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Spring, O.; Conrad, J.; Cook, L.W.; Palmquist, D.E.; Foley, M.E. Sesquiterpene lactone composition of wild and cultivated sunflowers and biological activity against an insect pest. J. Agric. Food Chem. 2015, 63, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, A.K.; Horakh, S.; Spring, O. Linear glandular trichomes of Helianthus (Asteraceae): Morphology, localization, metabolite activity and occurrence. AoB Plants 2013, 5, plt028. [Google Scholar] [CrossRef]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Li, N.; Che, Y.Y.; Zhang, Y.; Liang, S.X.; Zhao, M.B.; Jianga, Y.; Tu, P.F. Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Lopes, N.P.; Silva, D.B. Application of MALDI mass spectrometry in natural products analysis. Planta Med. 2016, 82, 671–689. [Google Scholar] [CrossRef] [PubMed]
- Spring, O.; Bienert, U.; Klemt, V. Sesquiterpene lactones in glandular trichomes of sunflower leaves. J. Plant Physiol. 1987, 130, 433–439. [Google Scholar] [CrossRef]
- Kaspar, S.; Peukert, M.; Svatos, A.; Matros, A.; Mock, H.P. MALDI-imaging mass spectrometry—An emerging technique in plant biology. Proteomics 2011, 11, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Kaletas, B.K.; van der Wiel, I.M.; Stauber, J.; Dekker, L.J.; Guzel, C.; Kros, J.M.; Luider, T.M.; Heeren, R.M.A. Sample preparation issues for tissue imaging by imaging MS. Proteomics 2009, 9, 2622–2633. [Google Scholar] [CrossRef] [PubMed]
- Grassl, J.; Taylor, N.L.; Millar, A.H. Matrix-assisted laser desorption/ionisation mass spectrometry imaging and its development for plant protein imaging. Plant Met. 2011, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.B.; Lopes, N.P. MALDI-MS of flavonoids: A systematic investigation of ionization and in-source dissociation mechanisms. J. Mass Spectrom. 2015, 50, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.A.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.A.; Alfatafta, A.A.; Harman, J.L.; Everett, S.L.; Serino, A.A. Feeding and toxic effects of floral sesquiterpene lactones, diterpenes, and phenolics from sunflower (Helianthus annuus L.) on Western Corn Rootworm. J. Agric. Food Chem. 1991, 39, 2293–2299. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in favonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Silva, D.B.; Turatti, I.C.; Gouveia, D.R.; Ernst, M.; Teixeira, S.P.; Lopes, N.P. Mass spectrometry of flavonoid vicenin-2, based sunlight barriers in Lychnophora species. Sci. Rep. 2014, 4, 4309. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brentan Silva, D.; Aschenbrenner, A.-K.; Lopes, N.P.; Spring, O. Direct Analyses of Secondary Metabolites by Mass Spectrometry Imaging (MSI) from Sunflower (Helianthus annuus L.) Trichomes. Molecules 2017, 22, 774. https://doi.org/10.3390/molecules22050774
Brentan Silva D, Aschenbrenner A-K, Lopes NP, Spring O. Direct Analyses of Secondary Metabolites by Mass Spectrometry Imaging (MSI) from Sunflower (Helianthus annuus L.) Trichomes. Molecules. 2017; 22(5):774. https://doi.org/10.3390/molecules22050774
Chicago/Turabian StyleBrentan Silva, Denise, Anna-Katharina Aschenbrenner, Norberto Peporine Lopes, and Otmar Spring. 2017. "Direct Analyses of Secondary Metabolites by Mass Spectrometry Imaging (MSI) from Sunflower (Helianthus annuus L.) Trichomes" Molecules 22, no. 5: 774. https://doi.org/10.3390/molecules22050774
APA StyleBrentan Silva, D., Aschenbrenner, A. -K., Lopes, N. P., & Spring, O. (2017). Direct Analyses of Secondary Metabolites by Mass Spectrometry Imaging (MSI) from Sunflower (Helianthus annuus L.) Trichomes. Molecules, 22(5), 774. https://doi.org/10.3390/molecules22050774