Modes of Cell Death Induced by Photodynamic Therapy Using Zinc Phthalocyanine in Lung Cancer Cells Grown as a Monolayer and Three-Dimensional Multicellular Spheroids
Abstract
:1. Introduction
2. Results
2.1. Nuclear Morphology
2.2. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
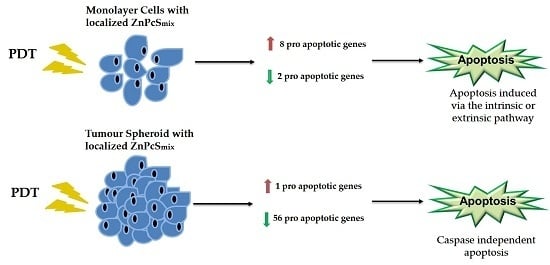
3. Discussion
3.1. Gene Expression Profiling in the Monolayer Cultures
3.2. Gene Expression Profiling in the MCTSs
4. Materials and Methods
4.1. Monolayer Cell Culture
4.2. MCTS Cell Culture
4.3. Photodynamic Treatment of Monolayer Cells
4.4. Photodynamic Treatment of MCTS
4.5. Nuclear Morphology
4.6. RNA Isolation and cDNA Synthesis
4.7. Gene Expression Profiling
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crous, A.; Abrahamse, H. High Fluence Low Intensity Laser Irradiation Bioinhibits Viability and Proliferation of Lung Cancer Stem Cells. J. Stem Cell Res. Ther. 2016. [Google Scholar] [CrossRef]
- Zago, G.; Muller, M.; van den Heuvel, M.; Baas, P. New targeted treatments for non-small-cell lung cancer—Role of nivolumab. Biologics 2016, 10, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ridge, C.A.; McErlean, A.M.; Ginsberg, M.S. Epidemiology of lung cancer. Semin. Interv. Radiol. 2013, 30, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25, 676, 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2014, 385, 977–1010. [Google Scholar] [CrossRef]
- Wu, K.; House, L.; Liu, W.; Cho, W. Personalized targeted therapy for lung cancer. Int. J. Mol. Sci. 2012, 13, 11471–11496. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Suda, K.; Wiens, J.; Bunn, P.A. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016, 388, 1012–1024. [Google Scholar] [CrossRef]
- Wan, M.T.; Lin, J.Y. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145–163. [Google Scholar] [CrossRef] [PubMed]
- De Paula, L.B.; Primo, F.L.; Pinto, M.R.; Morais, P.C.; Tedesco, A.C. Combination of hyperthermia and photodynamic therapy on mesenchymal stem cell line treated with chloroaluminum phthalocyanine magnetic-nanoemulsion. J. Magn. Magn. Mater. 2015, 380, 372–376. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Mori, S.; Suzuki, Y.; Kashiwagi, T.; Tokunaga, E.; Shibata, N.; Tanaka, M. Photodynamic Therapy Using Novel Zinc Phthalocyanine Derivatives and a Diode Laser for Superficial Tumors in Experimental Animals. J. Cancer Ther. 2014, 6, 53. [Google Scholar] [CrossRef]
- Raghavan, S.; Ward, M.R.; Rowley, K.R.; Wold, R.M.; Takayama, S.; Buckanovich, R.J.; Mehta, G. Formation of stable small cell number three dimensional ovarian cancer spheroids using hanging drop arrays for preclinical drug sensitivity assays. Gynecol. Oncol. 2015, 138, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Lin, C.; Cheng, J.; Su, J.; Zhao, H.; Liu, T.; Wen, X.; Zhao, P. Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLoS ONE 2015, 10, e0130348. [Google Scholar] [CrossRef] [PubMed]
- Manoto, S.L.; Houreld, N.N.; Abrahamse, H. Resistance of lung cancer cells grown as multicellular tumour spheroids to zinc sulfophthalocyanine photosensitization. Int. J. Mol. Sci. 2015, 16, 10185–10200. [Google Scholar] [CrossRef] [PubMed]
- Moscato, S.; Ronca, F.; Campani, D.; Danti, S. Poly (vinyl alcohol)/gelatin Hydrogels Cultured with HepG2 Cells as a 3D Model of Hepatocellular Carcinoma: A Morphological Study. J. Funct. Biomater. 2015, 6, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.I.; Ishida, S.; Hongo, T.; Ishikawa, Y.; Miyajima, A.; Sawada, J.I.; Ohno, Y.; Nakazawa, K.; Ozawa, S. Global gene expression changes including drug metabolism and disposition induced by three-dimensional culture of HepG2 cells-Involvement of microtubules. Biochem. Biophys. Res. Commun. 2009, 378, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Chen, M.; Gao, S.; Zhu, L. Activatable Ferritin Nanocomplex for Real-Time Monitoring of Caspase-3 Activation during Photodynamic Therapy. ACS Appl. Mater. Interfaces 2015, 7, 23248–23256. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Ferreira-Strixino, J.; Fontana, L.C.; Paula, L.M.; Raniero, L.; Martin, A.A.; Canevari, R.A. Apoptosis associated genes related to photodynamic therapy in breast carcinomas. Lasers Med. Sci. 2014, 29, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Manoto, S.L.; Houreld, N.N.; Abrahamse, H. Phototoxic effect of photodynamic therapy on lung cancer cells grown as a monolayer and three dimensional multicellular spheroids. Lasers Surg. Med. 2013, 45, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, L.C.; Casagrande, G.; Virador, V.M. In vitro three-dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2013, 52, 167–182. [Google Scholar]
- LaRocca, T.J. Programmed cell death dynamics during hyperglycemia and ischemic brain injuries. J. Neurol. Neurorehabil. Res. 2016, 1, 7–12. [Google Scholar]
- Vittar, N.B.R.; Awruch, J.; Azizuddin, K.; Rivarola, V. Caspase-independent apoptosis, in human MCF-7c3 breast cancer cells, following photodynamic therapy, with a novel water-soluble phthalocyanine. Int. J. Biochem. Cell Biol. 2010, 42, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, L.N.; Cheng, L.; Tu, S.; Guo, S.J.; Le, H.Y.; Xiong, Q.; Mo, R.; Li, C.Y.; Jeong, J.S.; et al. Bcl2-associated athanogene 3 interactome analysis reveals a new role in modulating proteasome activity. Mol. Cell. Proteom. 2013, 12, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; de Vasconcellos, J.F.; Sarkar, D.; Libermann, T.A.; Fisher, P.B.; Zerbini, L.F. GADD45 proteins: Central players in tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Bruemmer, D.; Blaschke, F.; Hsueh, W.A.; Law R, E.; van Herle, A.J. Signaling pathways involved in induction of GADD45 gene expression and apoptosis by troglitazone in human MCF-7 breast carcinoma cells. Oncogene 2004, 23, 4614–4623. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.; Majid, S.; Saini, S.; Zaman, M.S.; Yamamura, S.; Chiyomaru, T.; Shahryari, V.; Fukuhara, S.; Deng, G.; Dahiya, R.; et al. Hrk mediates 2-methoxyestradiol-induced mitochondrial apoptotic signaling in prostate cancer cells. Mol. Cancer Ther. 2013, 12, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deurse, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, C.; Chen, Y.; Zhou, T. Upregulation of acetylcholinesterase mediated by p53 contributes to cisplatin-induced apoptosis in human breast cancer cell. J. Cancer 2015, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sen, N.; Das, S. Tumour suppressor p53: Understanding the molecular mechanisms inherent to cancer. Curr. Sci. 2014, 107, 786. [Google Scholar]
- Karmakar, S.; Banik, N.L.; Patel, S.J.; Ray, S.K. 5-Aminolevulinic acid based photodynamic therapy suppressed survival factors and activated proteases for apoptosis in human glioblastoma U87MG cells. Neurosci. Lett. 2007, 415, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, X.; Kong, J. The proapoptotic protein BNIP3 interacts with VDAC to induce mitochondrial release of endonuclease G. PLoS ONE 2014, 9, e113642. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Searfoss, G.; Krolikowski, D.; Pagnoni, M.; Franks, C.; Clark, K.; Yu, K.T.; Jaye, M.; Ivashchenko, Y. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001, 8, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Lee, G.; Choi, H.; Myung, K.; Choi, Y. The expressions of TGF-β1 and IL10 in cultured fibroblasts after ALA-IPL photodynamic therapy. Ann. Dermatol. 2011, 23, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M. BCL2A1: The underdog in the BCL2 family. Cell Death Differ. 2012, 19, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kolluri, S.K.; Zhu, X.; Zhou, X.; Lin, B.; Chen, Y.; Sun, K.; Tian, X.; Town, J.; Cao, X.; Lin, F.; et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell 2008, 14, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Quinsay, M.N.; Thomas, R.L.; Lee, Y.; Gustafsson, A.B. Bnip3 mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy 2010, 6, 855–862. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Müller, R.D.; Oberholzer, A.; Zreiqat, H.; Kohl, B.; Ertel, W.; Hostmann, A.; Tschoeke, S.K.; Schulze-Tanzil, G. Interleukin-10 modulates pro-apoptotic effects of TNF-α in human articular chondrocytes in vitro. Cytokine 2007, 40, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, S.O.; Liu, X.; Owczarczak, B.; Musser, D.A.; Henderson, B.W. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997, 57, 3904–3909. [Google Scholar] [PubMed]
- Zeng, L.; Li, T.; Xu, D.C.; Liu, J.; Mao, G.; Cui, M.Z.; Fu, X.; Xu, X. Death receptor 6 induces apoptosis not through type I or type II pathways, but via a unique mitochondria-dependent pathway by interacting with Bax protein. J. Biol. Chem. 2012, 287, 29125–29133. [Google Scholar] [CrossRef] [PubMed]
- Benschop, R.; Wei, T.; Na, S. Tumor necrosis factor receptor superfamily member 21: TNFR-related death receptor-6, DR6. In Therapeutic Targets of the TNF Superfamily; Grewal, I.S., Ed.; Springer: New York, NY, USA, 2009; pp. 186–194. [Google Scholar]
Sample Availability: The human lung cancer cell line (A549, ATCC® CCL-185) is available from the ATCC. The ZnPcSmix was produced by the organic chemistry department. Both these are readily available. |



| Gene Symbol | Description | p Value | Fold Change |
|---|---|---|---|
| ABL1 | C-abl oncogene 1, non-receptor tyrosine kinase | 0.041 | 1.23 |
| BAG3 | BCL2-associated athanogene 3 | 0.006 | 1.36 |
| BAK1 | BCL2-antagonist/killer 1 | 0.033 | 1.41 |
| BCL2L10 | BCL2-like 10 (apoptosis facilitator) | 0.029 | 1.68 |
| BID | BH3 interacting domain death agonist | 0.035 | 1.20 |
| BIRC3 | Baculoviral IAP repeat containing 3 | 0.023 | −1.94 |
| CASP3 | Caspase 3, apoptosis-related cysteine peptidase | 0.036 | −1.70 |
| CASP5 | Caspase 5, apoptosis-related cysteine peptidase | 0.028 | 2.00 |
| CASP6 | Caspase 6, apoptosis-related cysteine peptidase | 0.045 | −1.16 |
| GADD45A | Growth arrest and DNA-damage-inducible, alpha | 0.005 | 1.43 |
| HRK | Harakiri, BCL2 interacting protein (contains only BH3 domain) | 0.026 | 1.96 |
| TNF | Tumour necrosis factor | 0.008 | 3.57 |
| TP53BP2 | Tumour protein p53 binding protein, 2 | 0.019 | 1.21 |
| Gene Symbol | Description | p Value | Fold Change |
|---|---|---|---|
| AKT1 | V-akt murine thymoma viral oncogene homolog 1 | 0.005 | −2.71 |
| APAF1 | Apoptotic peptidase activating factor 1 | 0.001 | −1.85 |
| BAD | BCL2-associated agonist of cell death | 0.001 | −1.55 |
| BAG1 | BCL2-associated athanogene | 0.034 | −1.15 |
| BAX | BCL2-associated X protein | 0.000 | −1.34 |
| BCL2 | B-cell CLL/lymphoma 2 | 0.041 | −1.37 |
| BCL2A1 | BCL2-related protein A1 | 0.022 | 1.52 |
| BCL2L1 | BCL2-like 1 | 0.004 | −1.42 |
| BCL2L11 | BCL2-like 11 (apoptosis facilitator) | 0.002 | −1.88 |
| BFAR | Bifunctional apoptosis regulator | 0.001 | −1.35 |
| BID | BH3 interacting domain death agonist | 0.004 | 1.10 |
| BIK | BCL2-interacting killer (apoptosis-inducing) | 0.038 | −1.33 |
| BIRC2 | Baculoviral IAP repeat containing 2 | 0.003 | −1.41 |
| BIRC5 | Baculoviral IAP repeat containing 5 | 0.003 | −1.45 |
| BIRC6 | Baculoviral IAP repeat containing 6 | 0.005 | −1.13 |
| BNIP2 | BCL2/adenovirus E1B 19 kDa interacting protein 2 | 0.010 | −1.44 |
| BNIP3 | BCL2/adenovirus E1B 19 kDa interacting protein 3 | 0.009 | 1.09 |
| BNIP3L | BCL2/adenovirus E1B 19 kDa interacting protein 3-like | 0.009 | −1.22 |
| BRAF | V-raf murine sarcoma viral oncogene homolog B1 | 0.001 | −1.48 |
| CASP10 | Caspase 10, apoptosis-related cysteine peptidase | 0.011 | −1.88 |
| CASP3 | Caspase 3, apoptosis-related cysteine peptidase | 0.012 | −1.31 |
| CASP4 | Caspase 4, apoptosis-related cysteine peptidase | 0.000 | −1.14 |
| CASP8 | Caspase 8, apoptosis-related cysteine peptidase | 0.002 | −1.55 |
| CASP9 | Caspase 9, apoptosis-related cysteine peptidase | 0.010 | −1.27 |
| CD27 | CD27 molecule | 0.003 | −2.14 |
| CD40 | CD40 molecule, TNF receptor superfamily member 5 | 0.006 | −1.31 |
| CD40LG | CD40 ligand | 0.022 | −1.80 |
| CD70 | CD70 molecule | 0.001 | −1.33 |
| CFLAR | CASP8 and FADD-like apoptosis regulator | 0.007 | −1.23 |
| CIDEA | Cell death-inducing DFFA-like effector a | 0.012 | −1.91 |
| CIDEB | Cell death-inducing DFFA-like effector b | 0.014 | −1.89 |
| CYCS | Cytochrome c, somatic | 0.002 | −1.92 |
| DAPK1 | Death-associated protein kinase 1 | 0.002 | −1.50 |
| DFFA | DNA fragmentation factor, 45 kDa, alpha polypeptide | 0.019 | −1.30 |
| DIABLO | Diablo, IAP-binding mitochondrial protein | 0.028 | −1.17 |
| FADD | Fas (TNFRSF6)-associated via death domain | 0.032 | −1.58 |
| FASLG | Fas ligand (TNF superfamily, member 6) | 0.023 | −2.03 |
| GADD45A | Growth arrest and DNA-damage-inducible, alpha | 0.034 | −1.36 |
| IGF1R | Insulin-like growth factor 1 receptor | 0.005 | −1.87 |
| IL10 | Interleukin 10 | 0.032 | −2.02 |
| LTBR | Lymphotoxin beta receptor (TNFR superfamily, member 3) | 0.001 | −1.15 |
| MCL1 | Myeloid cell leukemia sequence 1 (BCL2-related) | 0.000 | −1.35 |
| NAIP | NLR family, apoptosis inhibitory protein | 0.000 | −1.75 |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | 0.022 | −1.25 |
| NOL3 | Nucleolar protein 3 (apoptosis repressor with CARD domain) | 0.004 | −1.15 |
| TNFRSF10A | Tumour necrosis factor receptor superfamily, member 10a | 0.038 | −1.92 |
| TNFRSF10B | Tumour necrosis factor receptor superfamily, member 10b | 0.025 | −1.95 |
| TNFRSF1A | Tumour necrosis factor receptor superfamily, member 1A | 0.006 | −1.34 |
| TNFRSF25 | Tumour necrosis factor receptor superfamily, member 25 | 0.016 | −2.02 |
| TNFSF10 | Tumour necrosis factor (ligand) superfamily, member 10 | 0.000 | −2.10 |
| TNFSF8 | Tumour necrosis factor (ligand) superfamily, member 8 | 0.020 | −1.83 |
| TP53 | Tumour protein p53 | 0.045 | −1.42 |
| TP53BP2 | Tumour protein p53 binding protein, 2 | 0.001 | −1.21 |
| TP73 | Tumour protein p73 | 0.030 | −2.43 |
| TRADD | TNFRSF1A-associated via death domain | 0.001 | −1.52 |
| TRAF3 | TNF receptor-associated factor 3 | 0.038 | −1.26 |
| XIAP | X-linked inhibitor of apoptosis | 0.000 | −1.61 |
| Gene Symbol | Description | p Value | Fold Change |
|---|---|---|---|
| BAX | BCL2-associated X protein | 0.046 | −1.27 |
| BCL2L10 | BCL2-like 10 (apoptosis facilitator) | 0.034 | 1.09 |
| BNIP3 | BCL2/adenovirus E1B 19 kDa interacting protein 3 | 0.001 | 1.71 |
| BNIP3L | BCL2/adenovirus E1B 19 kDa interacting protein 3-like | 0.001 | 1.74 |
| CD40LG | CD40 ligand | 0.023 | 2.76 |
| FASLG | Fas ligand (TNF superfamily, member 6) | 0.030 | 3.52 |
| IL10 | Interleukin 10 | 0.000 | 3.54 |
| TNFRSF11B | Tumour necrosis factor receptor superfamily, member 11b | 0.004 | −1.56 |
| TNFRSF21 | Tumour necrosis factor receptor superfamily, member 21 | 0.022 | −1.20 |
| Parameters | Description/Value |
|---|---|
| Manufacturer | Oriel Corporation, USA |
| Wavelength (nm) | 680 |
| Wave emission | Continuous wave |
| Power output (mW) | 44.2 |
| Power density (mW/cm2) | 4.87 |
| Spot size (cm2) | 9.1 |
| Fluence (J/cm2) | 5 |
| Duration of exposure | 17 min 7 s |
| Pathway | Genes |
|---|---|
| Induction of apoptosis: | |
| Death domain receptors | CRADD, FADD, TNF, TNFRSF10B (DR5) |
| DNA damage | ABL1, CIDEA, CIDEB, TP53, TP73 |
| Extracellular signals | CFLAR (CASPER), DAPK1, TNFRSF25 (DR3) |
| Other | BAD, BAK1, BAX, BCL10, BCL2L11, BID, BIK, BNIP3, BNIP3L, CASP1 (ICE), CASP10 (MCH4), CASP14, CASP2, CASP3, CASP4, CASP6, CASP8, CD27 (TNFRSF7), CD70 (TNFSF7), CYCS, DFFA, DIABLO (SMAC), FAS (TNFRSF6), FASLG (TNFSF6), GADD45A, HRK, LTA (TNFB), NOD1 (CARD4), PYCARD (TMS1/ASC), TNFRSF10A, TNFRSF9, TNFSF10 (TRAIL), TNFSF8, TP53BP2, TRADD, TRAF3 |
| Anti-apoptosis: | AKT1, BAG1, BAG3, BAX, BCL2, BCL2A1 (Bfl-1/A1), BCL2L1 (BCL-X), BCL2L10, BCL2L2, BFAR, BIRC3 (c-IAP1), BIRC5, BIRC6, BNIP2, BNIP3, BNIP3L, BRAF, CD27 (TNFRSF7), CD40LG (TNFSF5), CFLAR (CASPER), DAPK1, FAS (TNFRSF6), HRK, IGF1R, IL10, MCL1, NAIP (BIRC1), NFKB1, NOL3, RIPK2, TNF, XIAP (BIRC4) |
| Regulation of apoptosis: | |
| Negative regulation | BAG1, BAG3, BCL10, BCL2, BCL2A1 (Bfl-1/A1), BCL2L1 (BCL-X), BCL2L10, BCL2L2, BFAR, BIRC2 (c-IAP2), BIRC3 (c-IAP1), BIRC6, BNIP2, BNIP3, BNIP3L, BRAF, CASP3, CD27 (TNFRSF7), CD40LG (TNFSF5), CFLAR (CASPER), CIDEA, DAPK1, DFFA, FAS (TNFRSF6), IGF1R, MCL1, NAIP (BIRC1), NOL3, TP53, TP73, XIAP (BIRC4) |
| Positive regulation | ABL1, AKT1, BAD, BAK1, BAX, BCL2L11, BID, BIK, BNIP3, BNIP3L, CASP1 (ICE), CASP10 (MCH4), CASP14, CASP2, CASP4, CASP6, CASP8, CD40 (TNFRSF5), CD70 (TNFSF7), CIDEB, CRADD, FADD, FASLG (TNFSF6), HRK, LTA (TNFB), LTBR, NOD1 (CARD4), PYCARD (TMS1/ASC), RIPK2, TNF, TNFRSF10A, TNFRSF10B (DR5), TNFRSF25 (DR3), TNFRSF9, TNFSF10 (TRAIL), TNFSF8, TP53, TP53BP2, TRADD, TRAF2, TRAF3 |
| Death domain proteins: | CRADD, DAPK1, FADD, TNFRSF10A, TNFRSF10B (DR5), TNFRSF11B, TNFRSF1A, TNFRSF1B, TNFRSF21, TNFRSF25 (DR3), TRADD |
| Caspases and regulators: | |
| Caspases | CASP1 (ICE), CASP10 (MCH4), CASP14, CASP2, CASP3, CASP4, CASP5, CASP6, CASP7, CASP8, CASP9, CFLAR (CASPER), CRADD, PYCARD (TMS1/ASC) |
| Caspase activators | AIFM1 (PDCD8), APAF1, BAX, BCL2L10, CASP1 (ICE), CASP9, NOD1 (CARD4), PYCARD (TMS1/ASC), TNFRSF10A, TNFRSF10B (DR5), TP53 |
| Caspase inhibitors | CD27 (TNFRSF7), XIAP (BIRC4) |
| Housekeeping genes: | ACTB, B2M, GAPDH, HPRT1, RPLP0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoto, S.L.; Houreld, N.; Hodgkinson, N.; Abrahamse, H. Modes of Cell Death Induced by Photodynamic Therapy Using Zinc Phthalocyanine in Lung Cancer Cells Grown as a Monolayer and Three-Dimensional Multicellular Spheroids. Molecules 2017, 22, 791. https://doi.org/10.3390/molecules22050791
Manoto SL, Houreld N, Hodgkinson N, Abrahamse H. Modes of Cell Death Induced by Photodynamic Therapy Using Zinc Phthalocyanine in Lung Cancer Cells Grown as a Monolayer and Three-Dimensional Multicellular Spheroids. Molecules. 2017; 22(5):791. https://doi.org/10.3390/molecules22050791
Chicago/Turabian StyleManoto, Sello L., Nicolette Houreld, Natasha Hodgkinson, and Heidi Abrahamse. 2017. "Modes of Cell Death Induced by Photodynamic Therapy Using Zinc Phthalocyanine in Lung Cancer Cells Grown as a Monolayer and Three-Dimensional Multicellular Spheroids" Molecules 22, no. 5: 791. https://doi.org/10.3390/molecules22050791
APA StyleManoto, S. L., Houreld, N., Hodgkinson, N., & Abrahamse, H. (2017). Modes of Cell Death Induced by Photodynamic Therapy Using Zinc Phthalocyanine in Lung Cancer Cells Grown as a Monolayer and Three-Dimensional Multicellular Spheroids. Molecules, 22(5), 791. https://doi.org/10.3390/molecules22050791