Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves
Abstract
:1. Introduction
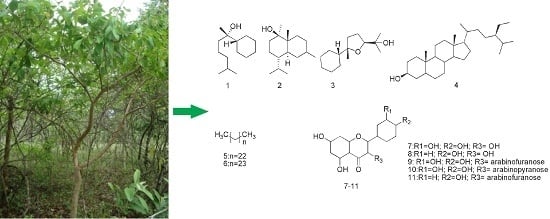
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. General Procedures
3.3. Extraction and Purification
3.4. Microbial Strains
3.5. In Vitro Susceptibility Testing
3.6. Structural Elucidation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Valli, M.; Santos, R.N.; Figueira, L.D.; Nakajima, C.H.; Castro-Gamboa, I.; Andricopulo, A.D.; Bolzani, V.S. Development of a natural products data base from the biodiversity of Brazil. J. Nat. Prod. 2013, 76, 439–444. [Google Scholar] [CrossRef] [PubMed]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Conceição, G.M.; Aragão, J.G. Diversidade e importância econômica das Myrtaceae do Cerrado, Parque Estadual do Mirador, Maranhão. Sci. Plena 2010, 6, 1–8. [Google Scholar]
- Gressler, E.; Pizo, M.A.; Morellato, L.P.C. Polinização e dispersão de sementes em Myrtaceae do Brasil. Braz. J. Bot. 2006, 29, 509–530. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Guilhon, G.M.S.P.; Andrade, E.H.A.; Zoghbi, M.G.B.; Santos, L.S. Constituents and pharmacological activities of Myrcia (Myrtaceae): A review of an aromatic and medicinal group of plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef] [PubMed]
- Judd, W.S.; Campbell, C.S.; Kellog, E.A.; Stevens, P.F.; Donoghe, M.J. Sistemática Vegetal: Um Enfoque Filogenético, 3rd ed.; Artmed: Porto Alegre, Brasil, 2009; p. 632. [Google Scholar]
- McVaugh, R. Myrtaceae, in the botany of the Guayana Highland, VIII. Mem. N. Y. Bot. Gard. 1969, 18, 55–286. [Google Scholar]
- Paula, J.E.; Imaña-Encinas, J.; Santana, O.A.; Ribeiro, G.S.; Imaña, C.R. Levantamento de floresta de galeria no ribeirão DoisIrmãos na APA de Cafuringa, DF, Brasil. Biotemas 2009, 22, 35–46. [Google Scholar]
- Silva, D.M.; Batalha, M.A. Defense syndromes against herbivory in a cerrado plant community. Plant Ecol. 2011, 212, 181–193. [Google Scholar] [CrossRef]
- Ferreira, A.C.F.; Neto, J.C.; Silva, A.C.M.; Kuster, R.M.; Carvalho, D.P. Inhibition of thyroid peroxidase by Myrcia uniflora flavonoids. Chem. Res. Toxicol. 2006, 19, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.A.; Gris, E.F.; Rebello, J.M.; Correia, J.F.G.; Oliveira, L.F.S.; Filho, D.W.; Pedrosa, R.C. The 2′,4′,6′-trihydroxyacetophenone isolated from Myrcia multiflora has antiobesity and mixed hypolipidemic effects with the reduction of lipid intestinal absorption. Planta Med. 2011, 77, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Vareda, P.M.P.; Saldanha, L.L.; Camaforte, N.A.D.P.; Violato, N.M.; Dokkedal, A.L.; Bosqueiro, J.R. Myrcia bella leaf extract presents hypoglycemic activity via PI3k/Akt insulin signaling pathway. Evid. Based Complement. Altern. Med. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yadegarinia, D.; Gachkar, L.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 2006, 67, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.S.; Guimarães, A.G.; Santana, M.T.; Siqueira, R.S.; Passos, L.O.; Machado, S.M.F.; Ribeiro, A.S.; Sobral, M.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Phytochemical screening, antinociceptive and anti-inflammatory effects of the essential oil of Myrcia pubiflora in mice. Rev. Bras. Farmacogn. 2012, 22, 181–188. [Google Scholar] [CrossRef]
- Xu, L.; Gao, J.; Wang, Y.; Yu, W.; Zhao, X.; Yang, X.; Zhong, Z.; Qian, Z.-M. Myrica rubra extracts protect the liver from CCl4-induced damage. Evidence-Based Complement. Altern. Med. 2011, 1–8. [Google Scholar]
- Correa-Royero, J.; Tangarife, V.; Durán, C.; Stashenko, E.; Mesa-Arango, A. In vitro antifungal activity and cytotoxic effect of essential oils and extracts of medicinal and aromatic plants against Candida krusei and Aspergillus fumigatus. Rev. Bras. Farmacogn. 2010, 20, 734–741. [Google Scholar] [CrossRef]
- Matos, M.E.; Sousa, M.P.; Matos, F.J.; Craveiro, A.A. Sesquiterpenes from Vanillosmopsis arborea. J. Nat. Prod. 1988, 51, 780–782. [Google Scholar] [CrossRef] [PubMed]
- NIST Standard Reference Database 1A NIST/EPA/NIH Mass Spectral Library (NIST 08) and NIST Mass Spectral Search Program; Version 2.0f; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008.
- Chaturvedula, V.S.P.; Prakash, I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242. [Google Scholar] [CrossRef]
- Goulart, M.; Sant’ana, A.; Lima, R.; Cavalcante, S.; Carvalho, M.; Filho, R. Fitoconstituintes químicos isolados de Jatropha elliptica. Atribuição dos deslocamentos químicos dos átomos de carbono e hidrogênio dos diterpenos Jatrofolonas A e B. Quim. Nova 1993, 16, 95–100. [Google Scholar]
- Chang, S.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Ryu, S.Y.; Lee, K.R. Phytochemical constituents of Bistorta manshuriensis. Nat. Prod. Sci. 2009, 15, 234–240. [Google Scholar]
- Begum, A.S.; Sahai, M.; Fujimoto, Y.; Asai, K.; Schneider, K.; Nicholson, G.; Suessmuth, R. A new kaempferol diglycoside from Datura suaveolens Humb. & Bonpl. ex. Willd. Nat. Prod. Res. 2006, 20, 1231–1236. [Google Scholar]
- Ignoato, M.C.; Fabrão, R.M.; Schuquel, I.T.A.; Botelho, M.F.P.; Santin, S.M.O.; Arruda, L.L.M.; Bersani-Amado, C.A.; Souza, M.C. Estudo fitoquímico e avaliação da atividade anti-inflamatória de Aeschynomene fluminensis vell. (Fabaceae). Quim. Nova 2012, 35, 2241–2244. [Google Scholar] [CrossRef]
- Chang, S.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Lee, K.R. Chemical constituents of Geranium eristemon. Nat. Prod. Sci. 2009, 15, 151–155. [Google Scholar]
- Prabu, G.R.; Gnanamani, A.; Sadulla, S. Guaijaverin—A plant flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol. 2006, 101, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.C.C.; Brandão, M.S.; Vieira-Júnior, G.M.; Menor, J.C.A.S.; Silva, H.B.; Soares, M.J.S.; Chaves, M.H. Atividade antibacteriana de plantas úteis e constituintes químicos da raiz de Copernicia prunifera. Rev. Bras. Farmacogn. 2008, 18, 90–97. [Google Scholar] [CrossRef]
- Regasini, L.O.; Pivatto, M.; Scorzoni, L.; Benaducci, T.; Fusco-Almeida, A.M.; Giannini, M.J.S.M.; Barreiro, E.J.; Siva, D.H.S.; Bolzani, V.S. Antimicrobial activity of Pterogyne nitens Tul., Fabaceae, against opportunistic fungi. Rev. Bras. Farmacogn. 2010, 20, 706–711. [Google Scholar] [CrossRef]
- Padovan, A.; Keszei, A.; Külheim, C.; Foley, W.J. The evolution of foliar terpene diversity in Myrtaceae. Phytochem. Rev. 2014, 13, 695–716. [Google Scholar] [CrossRef]
- Al-Ja’fari, A.-H.; Vila, R.; Freixa, B.; Tomi, F.; Casanova, J.; Costa, J.; Cañigueral, S. Composition and antifungal activity of the essential oil from the rhizome and roots of Ferula hermonis. Phytochemistry 2011, 72, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zeng, L.; Shi, G.; Zhao, G.-X.; Kozlowski, J.F.; McLaughlin, J.L. Bioactive compounds from Taiwania cryptomerioides. J. Nat. Prod. 1997, 60, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-C.; Ho, C.-L. Composition, in-vitro anticancer, and antimicrobial activities of the leaf essential oil of Machilus mushaensis from Taiwan. Nat. Prod. Commun. 2013, 8, 273–275. [Google Scholar] [PubMed]
- Kamatou, G.P.P.; Viljoen, A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Metwally, A.M.; Omar, A.A.; Harraz, F.M.; El Sohafy, S.M. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn. Mag. 2010, 6, 212–218. [Google Scholar] [PubMed]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Activity of phenolic compounds from plant origin against Candida species. Ind. Crops Prod. 2015, 74, 648–670. [Google Scholar] [CrossRef]
- Yun, J.; Lee, H.; Ko, H.J.; Woo, E.-R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim. Biophys. Acta 2015, 1848, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Aranda, R.; Granados-Guzmán, G.; Pérez-Meseguer, J.; González, G.; Torres, N. Activity of polyphenolic compounds against Candida glabrata. Molecules 2015, 20, 17903–17912. [Google Scholar] [CrossRef] [PubMed]
- Domingues, E.A.; Nakamura, C.V.; Souza, M.C.; Teixeira, T.S.; Peixoto, J.L.B.; Sarragiotto, M.H.; Vidotti, G.J. Estudo fitoquímico e avaliação da toxicidade frente a Artemia salina e da atividade antimicrobiana de Calycorectes psidiiflorus (O. Berg) Sobral, Myrtaceae. Rev. Bras. Farmacogn. 2010, 20, 23–27. [Google Scholar] [CrossRef]
- Paula, J.A.M.; Silva, M.R.R.; Costa, M.P.; Diniz, D.G.A.; Sá, F.A.S.; Alves, S.F.; Costa, É.A.; Lino, R.C.; Paula, J.R. Phytochemical analysis and antimicrobial, antinociceptive, and anti-inflammatory activities of two chemotypes of Pimenta pseudocaryophyllus (Myrtaceae). Evidence-Based Complement. Altern. Med. 2012, 2012, 1–15. [Google Scholar]
- Correia, A.F.; Silveira, D.; Fonseca-Bazzo, Y.M.; Magalhães, P.O.; Fagg, C.W.; Silva, E.C.; Gomes, S.M.; Gandolfi, L.; Pratesi, R.; Nóbrega, Y.K.M. Activity of crude extracts from Brazilian Cerrado plants against clinically relevant Candida species. BMC Complement. Altern. Med. 2016, 16, 203. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically, 8th ed.; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Information Supplement; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Microorganisms | EB | HF | DF | EAF | AF | Vanc. | Genta. | Fluc. | Itrac. |
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | |||||||||
| Bacillus cereus ATCC 14579 | 1000 | 250 | 250 | 1000 | 1000 | 2 | |||
| Bacillus subtilis ATCC 6633 | 1000 | 500 | 500 | 1000 | 1000 | 0.25 | |||
| Micrococcus luteus ATCC 9341 | 500 | 500 | 500 | 1000 | 1000 | 0.25 | |||
| Micrococcus roseus ATCC 1740 | 500 | 500 | 500 | 1000 | 1000 | 0.5 | |||
| Staphylococcus aureus ATCC 25923 | 750 | 500 | 500 | 1000 | 1000 | 1 | |||
| Staphylococcus aureus ATCC 6538 | 1000 | 250 | >1000 | 1000 | 1000 | 2 | |||
| Staphylococcus epidermidis ATCC 12229 | 1000 | 250 | 250 | 1000 | 1000 | 1 | |||
| Enterobacter aerogenes ATCC 13048 | >1000 | >1000 | >1000 | >1000 | >1000 | 0.125 | |||
| Enterobacter cloacae HMA/FTA502 | >1000 | >1000 | >1000 | >1000 | >1000 | 4 | |||
| Escherichia coli ATCC 11229 | >1000 | >1000 | >1000 | >1000 | >1000 | 2 | |||
| Escherichia coli ATCC 8739 | >1000 | 1000 | >1000 | >1000 | >1000 | 8 | |||
| Pseudomonas aeruginosa ATCC 27483 | 500 | 1000 | >1000 | 500 | 250 | 4 | |||
| Pseudomonas aeruginosa SPM1 | 500 | 1000 | 1000 | 250 | 125 | 4 | |||
| Salmonella sp ATCC 19430 | 1000 | >1000 | >1000 | 1000 | 1000 | 2 | |||
| Serratia marcescens ATCC 14756 | >1000 | >1000 | >1000 | >1000 | >1000 | 4 | |||
| Yeasts | |||||||||
| Candida albicans ATCC 90028 | 32 | 128 | 128 | 32 | 32 | 1 | |||
| Candida albicans 02 | 32 | 64 | 128 | 16 | 16 | 2 | |||
| Candida albicans 03 | 4 | 32 | 64 | 8 | 8 | >64 | |||
| Candida albicans 48 | 16 | 64 | 256 | 16 | 32 | >64 | |||
| Candida albicans 111 | 8 | 128 | 16 | 16 | 16 | >64 | |||
| Candida albicans 138 | 8 | 32 | 32 | 4 | 4 | 8 | |||
| Candida albicans 181 | 8 | 64 | 128 | 16 | 16 | >64 | |||
| Candida parapsilosis ATCC 22019 | 16 | 64 | 64 | 16 | 16 | 1 | |||
| Candida parapsilosis 11 | 16 | 64 | 64 | 16 | 16 | 1 | |||
| Cryptococcus neoformans ATCC 28957 | 16 | 16 | 32 | >1024 | >1024 | 2 | |||
| Cryptococcus gatti L1 | 16 | 16 | 32 | 1024 | 1024 | 2 | |||
| Cryptococcus neoformans L2 | 16 | 64 | 16 | 1024 | 1024 | 2 |
| Yeasts | Quercetin | Quercetin + Kaempferol | Avicularin | Juglanin | Guaijaverin |
|---|---|---|---|---|---|
| C. albicans | |||||
| ATCC 90028 | >128 | >128 | 16 | >128 | >128 |
| 02 | 64 | 128 | 4 | 128 | 16 |
| 03 | >128 | >128 | 8 | >128 | >128 |
| 48 | >128 | >128 | 8 | >128 | >128 |
| 111 | >128 | >128 | 8 | >128 | 128 |
| 138 | 128 | >128 | 2 | 16 | 2 |
| 181 | >128 | >128 | 4 | >128 | >128 |
| C. parapsilosis | |||||
| ATCC 22019 | >128 | >128 | 32 | 128 | 16 |
| 11 | 64 | 128 | 8 | 64 | 8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva Sa, F.A.; De Paula, J.A.M.; Dos Santos, P.A.; De Almeida Ribeiro Oliveira, L.; De Almeida Ribeiro Oliveira, G.; Liao, L.M.; De Paula, J.R.; Do Rosario Rodrigues Silva, M. Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves. Molecules 2017, 22, 1100. https://doi.org/10.3390/molecules22071100
Da Silva Sa FA, De Paula JAM, Dos Santos PA, De Almeida Ribeiro Oliveira L, De Almeida Ribeiro Oliveira G, Liao LM, De Paula JR, Do Rosario Rodrigues Silva M. Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves. Molecules. 2017; 22(7):1100. https://doi.org/10.3390/molecules22071100
Chicago/Turabian StyleDa Silva Sa, Fabyola Amaral, Joelma Abadia Marciano De Paula, Pierre Alexandre Dos Santos, Leandra De Almeida Ribeiro Oliveira, Gerlon De Almeida Ribeiro Oliveira, Luciano Morais Liao, Jose Realino De Paula, and Maria Do Rosario Rodrigues Silva. 2017. "Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves" Molecules 22, no. 7: 1100. https://doi.org/10.3390/molecules22071100
APA StyleDa Silva Sa, F. A., De Paula, J. A. M., Dos Santos, P. A., De Almeida Ribeiro Oliveira, L., De Almeida Ribeiro Oliveira, G., Liao, L. M., De Paula, J. R., & Do Rosario Rodrigues Silva, M. (2017). Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves. Molecules, 22(7), 1100. https://doi.org/10.3390/molecules22071100