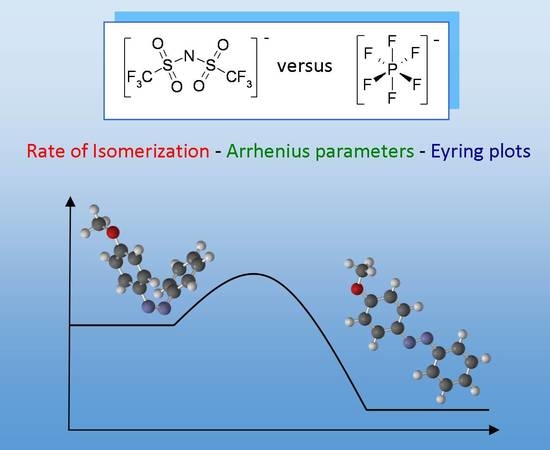
Kinetics and Energetics of Thermal Cis-Trans Isomerization of a Resonance-Activated Azobenzene in BMIM-Based Ionic Liquids for PF6−/Tf2N− Comparison
Abstract
:1. Introduction
2. Results
2.1. Thermal Cis-Trans Isomerization
2.2. Arrhenius and Eyring parameters
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
4.2. Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henzl, J.; Mehlorn, M.; Gawronski, H.; Rieder, K.H.; Morgenstern, K. Reversible cis-trans isomerization of a single azobenzene molecule. Angew. Chem. Int. Ed. 2006, 45, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Turansky, R.; Konopka, M.; Doltsinis, N.L.; Stich, I.; Marx, D. Switching of functionalized azobenzene suspended between gold tips by mechanochemical, photochemical, and opto-mechanical means. Phys. Chem. Chem. Phys. 2010, 12, 13922–13932. [Google Scholar] [CrossRef] [PubMed]
- Diau, E.W.G. A new trans to cis photoisomerization mechanism of azobenzene on the S1(n,π*) surface. J. Phys. Chem. A 2004, 108, 950–956. [Google Scholar] [CrossRef]
- Rau, H.; Lueddecke, E. On the rotation-inversion controversy on photoisomerization of azobenzenes. Experimental proof of inversion. J. Am. Chem. Soc. 1982, 104, 1616–1620. [Google Scholar] [CrossRef]
- Dias, A.R.; Minas de Piedade, M.E.; Martinho Simoes, J.A.; Simoni, J.A.; Teixeira, C.; Diogo, H.P.; Meng-Yan, Y.; Pilcher, G. Enthalpies of formation of cis-azobenzene and trans-azobenzene. J. Chem. Termodyn. 1992, 24, 439–447. [Google Scholar] [CrossRef]
- Cimiraglia, R.; Asano, T.; Hofmann, H.J. Mechanism of thermal Z/E isomerization of aromatic azo compounds. Relation between rotation and inversion states. Gazz. Chim. Ital. 1996, 126, 679–684. [Google Scholar]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- De Maria, P.; Fontana, A.; Gasbarri, C.; Siani, G.; Zanirato, P. Kinetics of the Z-E isomerization of monosubstituted azobenzenes in polar organic and aqueous micellar solvents. Arkivoc 2009, 8, 16–29. [Google Scholar] [CrossRef]
- Talaty, E.R.; Fargo, J.C. Thermal cis-trans-isomerization of substituted azobenzenes: a correction of the literature. Chem. Comm. 1967, 2, 65–66. [Google Scholar] [CrossRef]
- Herkstroeter, W. Mechanism of syn-anti isomerization of azomethine dyes. J. Am. Chem. Soc. 1973, 95, 8686–8691. [Google Scholar] [CrossRef]
- Angelini, G.; Canilho, N.; Emo, M.; Kingsley, M.; Gasbarri, C. Role of solvent and effect of substituent on azobenzene isomerization by using room-temperature ionic liquids as reaction media. J. Org. Chem. 2015, 80, 7430–7434. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Chiappe, C.; De Maria, P.; Fontana, A.; Gasparrini, F.; Pieraccini, D.; Pierini, M.; Siani, G. Determination of the polarities of some ionic liquids using 2-nitrocyclohexanone as the probe. J. Org. Chem. 2005, 70, 8193–8196. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J. On the solid, liquid and solution structural organization of imidazolium ionic liquids. Braz. Chem. Soc. 2004, 15, 341–350. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion effects on gas solubility in ionic liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef] [PubMed]
- Stolte, S.; Arning, J.; Bottin-Weber, U.; Matzke, M.; Stock, F.; Thiele, K.; Uerdingen, M.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Anion effects on the cytotoxicity of ionic liquids. Green Chem. 2006, 8, 621–629. [Google Scholar] [CrossRef]
- Latif, M.A.M.; Micaêlo, N.; Rahman, M.B.A. Solvation free energies in [bmim]-based ionic liquids: Anion effect toward solvation of amino acid side chain analogues. Chem. Phys. Lett. 2014, 615, 69–74. [Google Scholar] [CrossRef]
- Dos Santos, D.J.V.A.; Cordeiro, M.N.D.S. Effect of replacing [NTf2] by [PF6] anion on the [BMIM]-[NTf2] ionic liquid confined by gold. Mol. Simul. 2015, 41, 455–462. [Google Scholar] [CrossRef]
- Gasbarri, C.; Croce, F.; Meschini, I.; Bowen, C.H.; Marinelli, L.; Di Stefano, A.; Angelini, G. Single-walled carbon nanotubes in highly viscous media: A comparison between the dispersive agents [BMIM][BF4], L121, and Triton X-100. Chem. Eur. J. 2016, 22, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqmar, D.M.; Abdelkader, H.I.; Abou Kana, M.T.H. Imidazolium based ionic liquids as novel benign media for liquid-dye laser systems. J. Mol. Liq. 2017, 231, 370–378. [Google Scholar] [CrossRef]
- Hubbard, C.D.; Illner, P.; van Eldik, R. Understanding chemical reaction mechanisms in ionic liquids: Successes and challenges. Chem. Soc. Rev. 2011, 40, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Chiappe, C.; Pomelli, C.S. Computational studies on organic reactivity in ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Patrascu, C.; Gauffre, F.; Nallet, F.; Bordes, R.; Oberdisse, J.; de Lauth-Viguerie, N.; Mingotaud, C. Micelles in ionic liquids: Aggregation behavior of alkyl poly(ethyleneglycol)-ethers in 1-butyl-3-methyl-imidazolium type ionic liquids. Chem. Phys. Chem. 2006, 7, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Gasbarri, C. Polymeric aggregates in ionic liquids: the green future of the delivery systems. Curr. Drug Targets 2015, 16, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Ab Rani, M.A.; Brant, A.; Crowhurst, L.; Dolan, A.; Lui, M.; Hassan, N.H.; Hallett, J.P.; Hunt, P.A.; Niedermeyer, H.; Perez-Arlandis, J.M.; et al. Understanding the polarity of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
- Ue, M. Mobility and ionic association of lithium and quaternary ammonium salts in propylene carbonate and γ-butyrolactone. J. Electrochem. Soc. 1994, 141, 3336–3342. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, A.B.H.; Watanabe, M. Physicochemical properties and structures of room temperature ionic liquids. 1. Variation of anionic species. J. Phys. Chem. B 2004, 108, 16593–16600. [Google Scholar] [CrossRef]
- Schade, A.; Behme, N.; Spange, S. Dipolarity versus polarizability and acidity versus basicity of ionic liquids as a function of their molecular structures. Chem. Eur. J. 2014, 20, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Gasbarri, C. Solvent scales comparison by using α-nitrocyclohexanone as probe in ionic liquids, organic solvents and CH3CN/CHCl3 mixtures. Tetrahedron 2017, 73, 3036–3039. [Google Scholar] [CrossRef]
- Gasbarri, C.; Angelini, G. Polarizability over dipolarity for the spectroscopic behavior of azobenzenes in room-temperature ionic liquids and organic solvents. J. Mol. Liq. 2017, 229, 185–188. [Google Scholar] [CrossRef]
- Blevins, A.A.; Blanchard, G.J. Effect of positional substitution on the optical response of symmetrically disubstituted azobenzene derivatives. J. Phys. Chem. B 2004, 108, 4962–4968. [Google Scholar] [CrossRef]
- Whitten, D.G.; Wildes, P.D.; Pacifici, J.G.; Irick, G., Jr. Solvent and substituent on the thermal isomerization of substituted azobenzenes. Flash spectroscopic study. J. Am. Chem. Soc. 1971, 93, 2004–2008. [Google Scholar] [CrossRef]
- Dokić, J.; Gothe, M.; Wirth, J.; Peters, M.V.; Schwarz, J.; Hecht, S.; Saalfrank, P. Quantum chemical investigation of thermal cis-to-trans isomerization of azobenzene derivatives: Substituent effects, solvent effects, and comparison to experimental data. J. Phys. Chem. A 2009, 113, 6763–6773. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Ono, H.; Itoh, E.; Itoh, S.; Noda, K.; Usui, T.; Ishihara, K.; Inamo, M.; Takagi, H.D.; Asano, T. Kinetic study of thermal Z to E isomerization reactions of azobenzene and 4-dimethylamino-4'-nitroazobenzene in ionic liquids [1-R-3-methylimidazolium bis(trifluoromethylsulfonyl) imide with R = butyl, pentyl, and hexyl]. Chem. Eur. J. 2006, 12, 5328–5333. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.K.; Fuyuki, M.; Wada, A. Polarity controlled reaction path and kinetics of thermal cis-to-trans isomerization of 4-aminoazobenzene. J. Phys. Chem. B 2014, 118, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Okada, T.; Shinkai, S.; Shigematsu, K.; Kusano, Y.; Manabe, O. Temperature and pressure dependences of thermal cis-to-trans isomerization of azobenzenes which evidence an inversion mechanism. J. Am. Chem. Soc. 1981, 103, 5161–5165. [Google Scholar] [CrossRef]
- Asaka, T.; Akai, N.; Kawai, A.; Shibuya, K. Photochromism of 3-butyl-1-methyl-2-phenylazoimidazolium in room temperature ionic liquids. J. Photochem. Photobiol. A: Chem. 2010, 209, 12–18. [Google Scholar] [CrossRef]
- Crecca, C.R.; Roitberg, A.E. Theoretical study of the isomerization mechanism of azobenzene and disubstituted azobenzene derivatives. J. Phys. Chem. A 2006, 110, 8188–8203. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; De Paoli Lacerda, S.H.; Oliveira, D. Absorption of water by room-temperature ionic liquids: Effect of anions on concentration and state of water. Appl. Spectrosc. 2003, 57, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Gasbarri, C.; Angelini, G. Spectroscopic investigation of fluorinated phenols as pH-sensitive probes in mixed liposomal systems. RSC Adv. 2014, 4, 17840–17845. [Google Scholar] [CrossRef]
- Gasbarri, C.; Guernelli, S.; Boncompagni, S.; Angelini, G.; Siani, G.; De Maria, P.; Fontana, A. Fine-tuning of POPC liposomal leakage by the use of β-cyclodextrin and several hydrophobic guests. J. Liposome Res. 2010, 20, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Gasbarri, C.; Angelini, G.; Fontana, A.; De Maria, P.; Siani, G.; Giannicchi, I.; Della Cort, A. Kinetics of demetallation of a zinc-salophen complex into liposomes. Biochim. Biophys. Acta Biomembr. 2012, 1818, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Pisani, M.; Mobbili, G.; Marini, M.; Gasbarri, C. Neutral liposomes containing crown ether-lipids as potential DNA vectors. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Velluto, D.; Gasbarri, C.; Angelini, G.; Fontana, A. Use of simple kinetic and reaction-order measurements for the evaluation of the mechanism of surfactant-liposome interactions. J. Phys. Chem. B 2011, 115, 8130–8137. [Google Scholar] [CrossRef] [PubMed]
- De Maria, P.; Fontana, A.; Siani, G.; D’Aurizio, E.; Cerichelli, G.; Chiarini, M.; Angelini, G.; Gasbarri, C. Synthesis and aggregation behaviour of a new sultaine surfactant. Colloids Surf. B 2011, 87, 73–78. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of 4-methoxyazobenzene are available from the authors. |



| T (K) | BMIM PF6 10−6 kobs/s−1 | BMIM Tf2N 10−6 kobs/s−1 |
|---|---|---|
| 288 ± 0.1 | 1.19 ± 0.1 | 1.16 ± 0.2 |
| 298 ± 0.1 1 | 6.83 ± 0.1 | 4.19 ± 0.7 |
| 303 ± 0.1 | 8.42 ± 0.1 | 5.61 ± 0.1 |
| 313 ± 0.1 | 25.5 ± 0.2 | 24.1 ± 0.1 |
| 323 ± 0.1 | 129.6 ± 0.1 | 61.2 ± 0.1 |
| Ionic Liquid | Ea (kJ/mol) | A (s−1) | ΔH≠ (kJ/mol) | ΔS≠ (J/K mol) |
|---|---|---|---|---|
| BMIM PF6 | 98.4 ± 3.1 | (8.93 ± 2.5) × 1011 | 95.9 ± 3.0 | 70.3 ± 1.9 |
| BMIM Tf2N | 88.6 ± 2.8 | (0.13 ± 0.1) × 1011 | 86.0 ± 2.6 | 35.2 ± 1.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelini, G.; Campestre, C.; Scotti, L.; Gasbarri, C. Kinetics and Energetics of Thermal Cis-Trans Isomerization of a Resonance-Activated Azobenzene in BMIM-Based Ionic Liquids for PF6−/Tf2N− Comparison. Molecules 2017, 22, 1273. https://doi.org/10.3390/molecules22081273
Angelini G, Campestre C, Scotti L, Gasbarri C. Kinetics and Energetics of Thermal Cis-Trans Isomerization of a Resonance-Activated Azobenzene in BMIM-Based Ionic Liquids for PF6−/Tf2N− Comparison. Molecules. 2017; 22(8):1273. https://doi.org/10.3390/molecules22081273
Chicago/Turabian StyleAngelini, Guido, Cristina Campestre, Luca Scotti, and Carla Gasbarri. 2017. "Kinetics and Energetics of Thermal Cis-Trans Isomerization of a Resonance-Activated Azobenzene in BMIM-Based Ionic Liquids for PF6−/Tf2N− Comparison" Molecules 22, no. 8: 1273. https://doi.org/10.3390/molecules22081273
APA StyleAngelini, G., Campestre, C., Scotti, L., & Gasbarri, C. (2017). Kinetics and Energetics of Thermal Cis-Trans Isomerization of a Resonance-Activated Azobenzene in BMIM-Based Ionic Liquids for PF6−/Tf2N− Comparison. Molecules, 22(8), 1273. https://doi.org/10.3390/molecules22081273