Cytotoxicity of the Defensive Secretion from the Medicinal Insect Blaps rynchopetera
Abstract
:1. Introduction
2. Results and Discussion
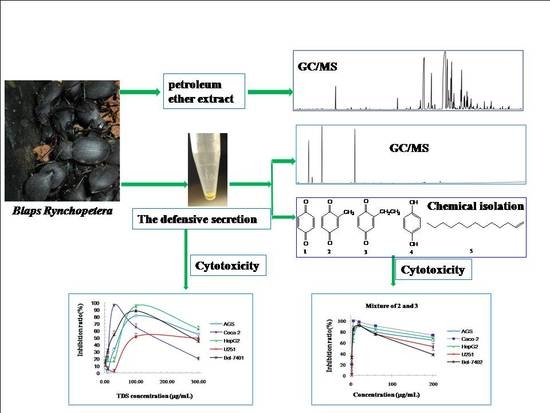
2.1. Cytotoxicity of TDS Against Human Cancer Cell Lines
2.2. Volatile Components Analysis of TDS
2.3. Isolation and Structural Identification Using NMR Spectrum
2.4. Constituent Comparison between TDS and Volatile Extract from Insect Bodies
2.5. Cytotoxicity of Isolated Compounds against Human Cancer Cell Lines
3. Experimental Section
3.1. Apparatus
3.2. Materials
3.3. Collection of Defensive Secretion
3.4. Extraction of the Volatile Extract
3.5. Cytotoxicity Assays
3.6. GC-MS Analysis
3.7. Isolation and Identification of Compounds in TDS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, X.H.; Wang, X.Z.; Jiang, H.L.; Yang, J.L.; Crews, P.; Valeriote, F.A.; Wu, Q.X. The biosynthetic products of Chinese insect medicine Aspongopus chinensis. Fitoterapia 2012, 83, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.A.; Mello, C.B.; Garcia, E.S.; But, T.M.; Azambuja, P. Insect natural products and processes: New treatments for human disease. Insect Biochem. Mol. Biol. 2011, 41, 747–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Tang, X.D.; Cheng, J.A. The Utilization and Industrialization of Insect Resources in China. Entomol. Res. 2008, 38, s38–s47. [Google Scholar] [CrossRef]
- Zhang, L.S.; Xia, C.L.; Yang, Y.S.; Liu, G.M. Progress on Studies of Blapsryn chopetera Fairmaire of the Yi Nationality. Lishizhen Med. Mater. Med. Res. 2009, 20, 3113–3114. [Google Scholar]
- Xiao, H.; Yin, T.P.; Dong, J.W.; Wu, X.M.; Luo, Q.; Luo, J.R.; Cai, L.; Ding, Z.T. Five New Phenolic Compounds with Antioxidant Activities from the Medicinal Insect Blaps rynchopetera. Molecules 2017, 22, 1301. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cai, L.; Wu, X.M.; Guo, N.N.; Luo, J.R.; Xiao, H. Cyclodipeptides from medicinal insect Blaps rynchopetera. Chin. Tradit. Herb. Drugs 2015, 46, 2381–2384. [Google Scholar]
- Shan, H.; Luo, Q.; Xiao, H. The Polyphenols Content Analysis of Blaps Rynchopetera Fairmaire. Lishizhen Med. Mater. Med. Res. 2016, 27, 532–534. [Google Scholar]
- Shi, G.R.; Zhuang, X.L.; Guo, M.X.; Wu, X.M.; Xiao, H. Study on the effect of in Vitro Antineoplastic Activity from the Extract of Blaps Rynchopetera Fairmaire. J. Anhui Agric. Sci. 2012, 40, 3387–3388. [Google Scholar]
- Yan, Y.M.; Li, L.J.; Qin, X.C.; Lu, Q.; Tu, Z.C.; Cheng, Y.X. Compounds from the insect Blaps japanensis with COX-1 and COX-2 inhibitory activities. Bioorg. Med. Chem. Lett. 2015, 25, 2469–2472. [Google Scholar]
- Yan, Y.M.; Dai, H.Q.; Du, Y.; Schneider, B.; Guo, H.; Li, D.P.; Cheng, Y.X. Identification of blapsins A and B as potent small-molecule 14-3-3 inhibitors from the insect Blaps japanensis. Bioorg. Med. Chem. Lett. 2012, 22, 4179–4181. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.M.; Qing, L.; Dong, X.P.; Yan, L.; Cheng, Y.X. Chemical Constituents from the Insect Blaps japonensis. Nat. Prod. Res. Dev. 2012, 24, 1712–1714. [Google Scholar]
- Wahrendorf, M.S.; Wink, M. Pharmacologically active natural products in the defences ecretion of Palembus ocularis (Tenebrionidae, Coleoptera). J. Ethnopharmacol. 2006, 106, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.R.; Zhuang, X.L.; Geng, L.; Wu, X.M.; Luo, J.R.; Liu, G.M. In Vitro Antibacterial Activity of Extracts from Blaps rynchopetera Fairmair. J. Dali Univ. 2012, 11, 5–7. [Google Scholar]
- Cui, W.B.; Qian, J.F.; Luo, J.R.; Xiao, H. Antioxidant Activity of Solvent Extracts of Blaps rynchopetera Fairmaire in Vitro. Lishizhen Med. Mater. Med. Res. 2013, 24, 1566–1567. [Google Scholar]
- Peschke, K.; Eisner, T. Defensive secretion of the tenebrionid beetle, Blaps mucronata: Physical and chemical determinants of effectiveness. J. Comp. Physiol. 1987, 161, 377–388. [Google Scholar] [CrossRef]
- Gross, J.; Schumacher, K.; Schmidtberg, H.; Vilcinskas, A. Protected by fumigants: beetle perfumes in antimicrobial defense. J. Chem. Ecol. 2008, 34, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, M.L.; Girotti, J.R.; Mijailovsky, S.J.; Pedrini, N.; Juárez, M.P. Volatile secretions and epicuticular hydrocarbons of the beetle Ulomoides dermestoides. Comp. Biochem. Physiol. B 2009, 154, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Gasch, T.; Schott, M.; Wehrenfennig, C.; Düring, R.A.; Vilcinskas, A. Multifunctional weaponry: The chemical defenses of earwigs. J. Insect Physiol. 2013, 59, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.R.; He, J.B.; Yang, Y.S.; Liu, G.M. Study on Chemical Constituents in medicinal insect Blaps rynchopetera Fairmaire. Chin. Tradit. Pat. Med. 2010, 32, 2013–2014. [Google Scholar]
- Shen, C.C.; Lin, C.-F.; Huang, Y.L.; Wan, S.T.; Chen, C.-C.; Sheu, S.J.; Lin, Y.C.; Chen, C.C. Bioactive Components from the Mycelium of Antrodia Salmonea. J. Chin. Chem. Soc. 2008, 55, 854–857. [Google Scholar]
- Paul, D.J.; Laure, N.B.; Guru, S.K.; Khan, I.A.; Ajit, S.K.; Vishwakarma, R.A.; Pierre, T. Antiproliferative and antimicrobial activities of alkylbenzoquinone derivatives from Ardisia kivuensis. Pharm. Biol. 2014, 52, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.K.; Chon, S.K.; Kim, S.H.; Jeong, S.Y.; Kim, M.S.; Cho, S.H.; Lee, H.B.; Khang, G. Controlled release of paclitaxel from microemulsion containing PLGA and evaluation of anti-tumor activity in vitro and in vivo. Int. J. Pharm. 2004, 286, 147–156. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |





| Compounds | IC50 (μg/mL)/[± Standard Deviation, n = 3] | ||||
|---|---|---|---|---|---|
| AGS | Caco-2 | HepG2 | U251 | Bel-7402 | |
| cis-platinum (DDP) | 2.2 ± 0.3 | 1.6 ± 0.3 | 2.0 ±0.2 | 1.0 ± 0.1 | 1.7 ± 0.1 |
| mitoxantrone (MA) | 2.0 ± 0.2 | 2.6 ± 0.3 | 7.7 ± 0.3 | 0.9 ± 0.1 | 5.8 ± 0.9 |
| defensive secretion | 45.8 ± 5.9 | 17.4 ± 2.0 | 53.6 ± 5.6 | 98.4 ± 4.8 | 23.4 ± 1.2 |
| 1 (bought sample) | 12.8 ± 1.1 | 10.2 ± 1.3 | 23.1 ± 4.2 | 36.5 ± 5.4 | 11.6 ± 0.9 |
| mixture of 2 and 3 (about 1:3) | 3.6 ± 0.6 | 2.8 ± 0.5 | 3.8 ± 0.4 | 3.7 ± 0.8 | 2.8 ± 0.2 |
| 4 | 23.9 ± 0.9 | 10.8 ± 0.5 | 33.5 ± 2.1 | 44.2 ± 3.9 | 16.1 ± 0.8 |
| No. | Retention Time (min) | Content (%) | Compound | Molecular Formula | Molecular Weight | Similarity |
|---|---|---|---|---|---|---|
| 1 | 3.302 | 1.01 | p-benzoquinone | C6H4O2 | 108 | 95 |
| 2 | 4.684 | 22.86 | 2-methyl-2,5-cyclohexadiene-1,4-dione | C7H6O2 | 122 | 97 |
| 3 | 5.161 | 3.51 | Not identified | |||
| 4 | 6.484 | 31.00 | 2-ethyl-2,5-cyclohexadiene-1,4-dione | C8H8O2 | 136 | 93 |
| 5 | 10.394 | 1.33 | Hydroquinone | C6H6O2 | 110 | 92 |
| 6 | 10.943 | 28.02 | 1-tridecene | C13H26 | 182 | 98 |
| 7–15 | 12.27 | Not identified | ||||
| Total | 100.00 | |||||
| Total identified | 84.22 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Dong, J.-W.; Zhou, D.-J.; Wu, X.-M.; Luo, J.-R.; Zhang, C.-G.; Guo, N.-N.; Li, Y.; Cai, L.; Ding, Z.-T. Cytotoxicity of the Defensive Secretion from the Medicinal Insect Blaps rynchopetera. Molecules 2018, 23, 10. https://doi.org/10.3390/molecules23010010
Xiao H, Dong J-W, Zhou D-J, Wu X-M, Luo J-R, Zhang C-G, Guo N-N, Li Y, Cai L, Ding Z-T. Cytotoxicity of the Defensive Secretion from the Medicinal Insect Blaps rynchopetera. Molecules. 2018; 23(1):10. https://doi.org/10.3390/molecules23010010
Chicago/Turabian StyleXiao, Huai, Jian-Wei Dong, Di-Jiao Zhou, Xiu-Mei Wu, Jian-Rong Luo, Cheng-Gui Zhang, Na-Na Guo, Yue Li, Le Cai, and Zhong-Tao Ding. 2018. "Cytotoxicity of the Defensive Secretion from the Medicinal Insect Blaps rynchopetera" Molecules 23, no. 1: 10. https://doi.org/10.3390/molecules23010010
APA StyleXiao, H., Dong, J. -W., Zhou, D. -J., Wu, X. -M., Luo, J. -R., Zhang, C. -G., Guo, N. -N., Li, Y., Cai, L., & Ding, Z. -T. (2018). Cytotoxicity of the Defensive Secretion from the Medicinal Insect Blaps rynchopetera. Molecules, 23(1), 10. https://doi.org/10.3390/molecules23010010