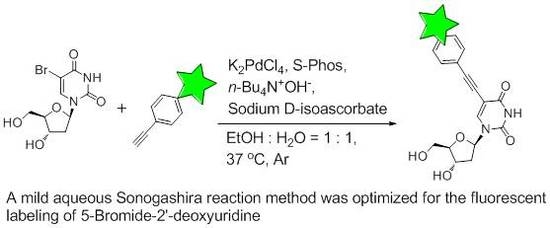
A Mild Aqueous Sonogashira Reaction as a Fluorescent Labeling Strategy for 5-Bromide-2′-Deoxyuridine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of Various Aliphatic or Aryl Alkyne-Containing Fluorescent Dyes
2.2. Optimization of Sonogashira Reaction Conditions
2.3. Labeling BrdU with Alkyne-Containing Fluorescent Dyes
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 3′,6′-Bis(diethylamino)-2-(3-ethynylphenyl)spiro[isoindoline-1,9′-xanthen]-3-one (1)
3.3. Synthesis of 2-(4-(But-3-yn-1-yloxy)butyl)isoindoline-1,3-dione (9)
3.4. Synthesis of 2-(4-(But-3-yn-1-yloxy)butyl)-3′,6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one (2)
3.5. Synthesis of 7-(Diethylamino)-N-(3-ethynylphenyl)-2-oxo-2H-chromene-3-carboxamide (3)
3.6. Synthesis of 7-(Diethylamino)-2-oxo-N-(prop-2-yn-1-yl)-2H-chromene-3-carboxamide (4)
3.7. The Protocol of Base Optimization
3.8. The Protocol of Catalytic System Optimization
3.9. Synthesis of 5-((3-(3′,6′-Bis(diethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)phenyl)ethynyl)-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione (12)
3.10. Synthesis of 5-(4-(4-(3′,6′-Bis(diethylamino)-3-oxospiro[isoindoline-1,9′-xanthen]-2-yl)butoxy)but-1-yn-1-yl)-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione (13)
3.11. 7-(Diethylamino)-N-(3-((1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)ethynyl)phenyl)-2-oxo-2H-chromene-3-carboxamide (14)
3.12. 7-(Diethylamino)-N-(3-(1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)prop-2-yn-1-yl)-2-oxo-2H-chromene-3-carboxamide (15)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cavanagh, B.L.; Walker, T.; Norazit, A.; Meedeniya, A.C. Thymidine analogues for tracking DNA synthesis. Molecules 2011, 16, 7980–7993. [Google Scholar] [CrossRef] [PubMed]
- Kore, A.R.; Charles, I. Recent Developments in the Synthesis and Applications of C5-Substituted Pyrimidine Nucleosides and Nucleotides. Curr. Org. Chem. 2012, 16, 1996–2013. [Google Scholar] [CrossRef]
- Gratzner, H.G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science 1982, 218, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Salic, A.; Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Buck, S.B.; Bradford, J.; Gee, K.R.; Agnew, B.J.; Clarke, S.T.; Salic, A. Detection of S-phase cell cycle progression using 5-ethynyl-2′-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2′-deoxyuridine antibodies. BioTechniques 2008, 44, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Rieder, U.; Luedtke, N.W. Alkene-tetrazine ligation for imaging cellular DNA. Angew. Chem. 2014, 53, 9168–9172. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; El-Sagheer, A.H.; Brown, T. Azide and trans-cyclooctene dUTPs: Incorporation into DNA probes and fluorescent click-labelling. Analyst 2015, 140, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Neef, A.B.; Luedtke, N.W. An azide-modified nucleoside for metabolic labeling of DNA. Chembiochem 2014, 15, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.M.; Kee, N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 2006, 1, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Liboska, R.; Ligasova, A.; Strunin, D.; Rosenberg, I.; Koberna, K. Most anti-BrdU antibodies react with 2′-deoxy-5-ethynyluridine—The method for the effective suppression of this cross-reactivity. PLoS ONE 2012, 7, e51679. [Google Scholar] [CrossRef] [PubMed]
- Kitao, H.; Morodomi, Y.; Niimi, S.; Kiniwa, M.; Shigeno, K.; Matsuoka, K.; Kataoka, Y.; Iimori, M.; Tokunaga, E.; Saeki, H.; et al. The antibodies against 5-bromo-2′-deoxyuridine specifically recognize trifluridine incorporated into DNA. Sci. Rep. 2016, 6, 25286. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, K.; Hossain, M.J.; Roberti, M.J.; Ellenberg, J.; Hirota, T. Sister chromatid resolution is an intrinsic part of chromosome organization in prophase. Nat. Cell Biol. 2016, 18, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.J.; Peterson, D.A. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat. Methods 2005, 2, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Lercher, L.; McGouran, J.F.; Kessler, B.M.; Schofield, C.J.; Davis, B.G. DNA modification under mild conditions by Suzuki-Miyaura cross-coupling for the generation of functional probes. Angew. Chem. 2013, 52, 10553–10558. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, T.; Littke, A.F.; Buchwald, S.L.; Fu, G.C. Pd(PhCN)(2)Cl(2)/P(t-Bu)(3): A versatile catalyst for Sonogashira reactions of aryl bromides at room temperature. Org. Lett. 2000, 2, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, M.; Doucet, H.; Santelli, M. Coupling reactions of aryl bromides with 1-alkynols catalysed by a tetraphosphine/palladium catalyst. Tetrahedron Lett. 2004, 45, 1603–1606. [Google Scholar] [CrossRef]
- Komáromi, A.; Tolnai, G.L.; Novák, Z. Copper-free Sonogashira coupling in amine–water solvent mixtures. Tetrahedron Lett. 2008, 49, 7294–7298. [Google Scholar] [CrossRef]
- Zhang, J.; Đaković, M.; Popović, Z.; Wu, H.; Liu, Y. A functionalized ionic liquid containing phosphine-ligated palladium complex for the Sonogashira reactions under aerobic and CuI-free conditions. Catal. Commun. 2012, 17, 160–163. [Google Scholar] [CrossRef]
- Liang, B.; Dai, M.; Chen, J.; Yang, Z. Copper-free sonogashira coupling reaction with PdCl2 in water under aerobic conditions. J. Org. Chem. 2005, 70, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Chung, D.W.; Rich, B. Sonogashira couplings of aryl bromides: room temperature, water only, no copper. Org. Lett. 2008, 10, 3793–3796. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.; Shinmen, M.; Nishitani, S.; Sato, M.; Ryu, I. A copper-free Sonogashira coupling reaction in ionic liquids and its application to a microflow system for efficient catalyst recycling. Org. Lett. 2002, 4, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Cui, H.; Jin, J.; Lai, F.; Wen, H.; Zhang, X.; Ruda, G.F.; Chen, X.; Yin, D. Development of 3-alkyl-6-methoxy-7-hydroxy-chromones (AMHCs) from natural isoflavones, a new class of fluorescent scaffolds for biological imaging. Chem. Commun. 2015, 51, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, M.; Zhai, D.; Er, J.C.; Chang, Y.T. Combinatorial strategies in fluorescent probe development. Chem. Rev. 2012, 112, 4391–4420. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Cui, Q.; Meng, H.; Lai, F.; Wang, S.; Zhang, X.; Chen, X.; Cui, H.; Yin, D. A high-resolution method to assess cell multinucleation with cytoplasm-localized fluorescent probes. Analyst 2016, 141, 4010–4013. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Shen, Z.; Wen, H.; Chen, J.; Zhang, X.; Lin, P.; Yin, D.; Cui, H.; Chen, X. A Morphological identification cell cytotoxicity assay using cytoplasm-localized fluorescent probe (CLFP) to distinguish living and dead cells. Biochem. Biophys. Res. Commun. 2017, 482, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.T.; Khan, A.U. L-ascorbic acid quenching of singlet delta molecular oxygen in aqueous media: Generalized antioxidant property of vitamin C. Biochem. Biophys. Res. Commun. 1983, 115, 932–937. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |







| Base | Yield (%) |
|---|---|
| Na2CO3 | 19 |
| Cs2CO3 | 21 |
| Et3N | 28 |
| Quinine | 34 |
| DABCO | 39 |
| n-Bu4N+OH− | 42 |
| The Catalyst | The Solvent | Yield |
|---|---|---|
| Pd(OAc)2, DTBPPS, CuI | DMF/H2O = 1:1 | 22% |
| K2PdCl4, DTBPPS, CuI | DMF/H2O = 1:1 | 42% |
| K2PdCl4, S-phos | EtOH/H2O = 1:1 | 75% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Gao, Y.; Shen, S.; Wen, H.; Cui, H. A Mild Aqueous Sonogashira Reaction as a Fluorescent Labeling Strategy for 5-Bromide-2′-Deoxyuridine. Molecules 2018, 23, 154. https://doi.org/10.3390/molecules23010154
Wang S, Gao Y, Shen S, Wen H, Cui H. A Mild Aqueous Sonogashira Reaction as a Fluorescent Labeling Strategy for 5-Bromide-2′-Deoxyuridine. Molecules. 2018; 23(1):154. https://doi.org/10.3390/molecules23010154
Chicago/Turabian StyleWang, Shufang, Yongxin Gao, Shigang Shen, Hui Wen, and Huaqing Cui. 2018. "A Mild Aqueous Sonogashira Reaction as a Fluorescent Labeling Strategy for 5-Bromide-2′-Deoxyuridine" Molecules 23, no. 1: 154. https://doi.org/10.3390/molecules23010154
APA StyleWang, S., Gao, Y., Shen, S., Wen, H., & Cui, H. (2018). A Mild Aqueous Sonogashira Reaction as a Fluorescent Labeling Strategy for 5-Bromide-2′-Deoxyuridine. Molecules, 23(1), 154. https://doi.org/10.3390/molecules23010154