Chiral and Molecular Recognition through Protonation between Aromatic Amino Acids and Tripeptides Probed by Collision-Activated Dissociation in the Gas Phase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chiral Recognition Ability of l-Alanine Peptides
2.2. Chiral and Molecular Recognition of l-Serine-Containing Tripeptides
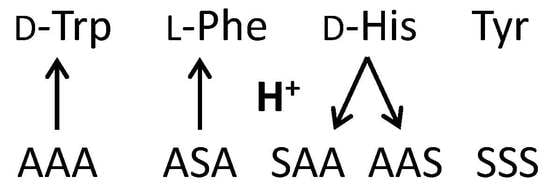
2.2.1. Tryptophan and Phenylalanine
2.2.2. Tyrosine and Histidine
2.2.3. Serine Tripeptide
2.3. Chiral Recognition and Enantiomeric Excess Formation in Molecular Clouds
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Srinivas, N.R. Evaluation of experimental strategies for the development of chiral chromatographic methods based on diastereomer formation. Biomed. Chromatogr. 2004, 18, 207–233. [Google Scholar] [CrossRef] [PubMed]
- McConnell, O.; Bach, A.; Balibar, C.; Byrne, N.; Cai, Y.; Carter, G.; Chlenov, M.; Di, L.; Fan, K.; Goljer, I.; et al. Enantiomeric separation and determination of absolute stereochemistry of asymmetric molecules in drug discovery-Building chiral technology toolboxes. Chirality 2007, 19, 658–682. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Machida, Y.; Nishi, H.; Kamigauchi, M.; Minoura, K.; Ishida, T. Structural requirement for chiral recognition of amino acid by (18-crown-6)-tetracarboxylic acid: Binding analysis in solution and solid states. Bull. Chem. Soc. Jpn. 2009, 82, 219–229. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral recognition mechanisms in analytical separation sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Sawada, M. Chiral recognition detected by fast atom bombardment mass spectrometry. Mass Spectrom. Rev. 1997, 16, 73–90. [Google Scholar] [CrossRef]
- Awad, H.; El-Aneed, A. Enantioselectivity of mass spectrometry: Challenges and promises. Mass Spectrom. Rev. 2013, 32, 466–483. [Google Scholar] [CrossRef] [PubMed]
- Piovesana, S.; Samperi, R.; Lagana, A.; Bella, M. Determination of enantioselectivity and enantiomeric excess by mass spectrometry in the absence of chiral chromatographic separation: An overview. Chem. Eur. J. 2013, 19, 11478–11494. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yao, Z.P. Chiral recognition and determination of enantiomeric excess by mass spectrometry: A review. Anal. Chim. Acta 2017, 968, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Faggi, E.; Vicent, C.; Luis, S.V.; Alfonso, I. Stereoselective recognition of the Ac-Glu-Tyr-OH dipeptide by pseudopeptidic cages. Org. Biomol. Chem. 2015, 13, 11721–11731. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Wu, C.; Matz, L.M.; Clowers, B.H.; Siems, W.F.; Hill, H.H., Jr. Gas-phase chiral separations by ion mobility spectrometry. Anal. Chem. 2006, 78, 8200–8206. [Google Scholar] [CrossRef] [PubMed]
- Domalain, V.; Hubert-Roux, M.; Tognetti, V.; Joubert, L.; Lange, C.M.; Rouden, J.; Afonso, C. Enantiomeric differentiation of aromatic amino acids using traveling wave ion mobility-mass spectrometry. Chem. Sci. 2014, 5, 3234–3239. [Google Scholar] [CrossRef]
- Yu, X.; Yao, Z.P. Chiral differentiation of amino acids through binuclear copper bound tetramers by ion mobility mass spectrometry. Anal. Chim. Acta 2017, 981, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Fuke, K.; Tona, M.; Fujihara, A.; Sakurai, M.; Ishikawa, H. Design and development of a novel nuclear magnetic resonance detection for the gas phase ions by magnetic resonance acceleration technique. Rev. Sci. Instrum. 2012, 83, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fuke, K.; Ohshima, Y.; Tona, M. Preparation of cold ions in strong magnetic field and its application to gas-phase NMR spectroscopy. Hyperfine Interact. 2015, 236, 9–18. [Google Scholar] [CrossRef]
- Bonner, W.A. The origin and amplification of biomolecular chirality. Orig. Life Evol. Biosph. 1991, 21, 59–111. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.; Marcellus, P.D.; d’Hendecourt, L.L.S.; Nahon, L.; Jones, N.C.; Hoffmann, S.V.; Bredehöft, J.H.; Meierhenrich, U.J. Photochirogenesis: Photochemical models on the absolute asymmetric formation of amino acids in interstellar space. Phys. Life Rev. 2011, 8, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mirazo, K.; Briones, C.; Escosura, A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbier, B.; Segovia, A.A.; Rosenbauer, H.; Thiemann, W.H.P.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Gontareva, N.B.; Kuzicheva, E.A.; Shelegedin, V.N. Synthesis and characterization of peptides after high-energy impact on the icy matrix: Preliminary step for further UV-induced formation. Plan. Space Sci. 2009, 57, 441–445. [Google Scholar] [CrossRef]
- Kaiser, R.I.; Stockton, A.M.; Kim, Y.S.; Jensen, E.C.; Mathies, R.A. On the formation of dipeptides in interstellar model ices. Astrophys. J. 2013, 765, 111–119. [Google Scholar] [CrossRef]
- Abplanalp, M.J.; Förestel, M.; Kaiser, R.I. Exploiting single photon vacuum ultraviolet photoionization to unravel the synthesis of complex organic molecules in interstellar ices. Chem. Phys. Lett. 2016, 644, 79–98. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.H.; Macko, S.A. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 1997, 389, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Groy, T.L. Molecular asymmetry in extraterrestrial organic chemistry: An analytical perspective. Geochim. Cosmochim. Acta 2011, 75, 645–656. [Google Scholar] [CrossRef]
- McGuire, B.A.; Carroll, P.B.; Loomis, R.A.; Finneran, I.A.; Jewell, P.R.; Remijan, A.J.; Blake, G.A. Discovery of the interstellar chiral molecule propylene oxide (CH3CHCH2O). Science 2016, 352, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Ménard, F.; Tamura, M. Circular polarization in star-formation regions: Implications for biomolecular homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Sato, T.; Hayakawa, S. Enantiomer-selective ultraviolet photolysis of temperature-controlled protonated tryptophan on a chiral crown ether in the gas phase. Chem. Phys. Lett. 2014, 610–611, 228–233. [Google Scholar] [CrossRef]
- Fujihara, A.; Maeda, N.; Hayakawa, S. Quantitative chiral analysis of tryptophan using enantiomer-selective photolysis of cold non-covalent complexes in the gas phase. J. Mass Spectrom. 2015, 50, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Maeda, N.; Hayakawa, S. Enantiomer-selective photolysis of cold gas-phase tryptophan in l -serine clusters with linearly polarized light. Orig. Life Evol. Biosph. 2014, 44, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.N.; Fujihara, A. Enantiomer-selective photo-induced reaction of protonated tryptophan with disaccharides in the gas phase. Orig. Life Evol. Biosph. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Maeda, N.; Doan, T.N.; Hayakawa, S. Enantiomeric excess determination for monosaccharides using chiral transmission to cold gas-phase tryptophan in ultraviolet photodissociation. J. Am. Soc. Mass Spectrom. 2017, 28, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Maeda, N. Quantitative chiral analysis of amino acids in solution using enantiomer-selective photodissociation of cold gas-phase tryptophan via chiral recognition. Anal. Chim. Acta 2017, 979, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Matsuyama, H.; Tajiri, M.; Wada, Y.; Hayakawa, S. Enantioselective collision-activated dissociation of gas-phase tryptophan induced by chiral recognition of protonated l-alanine peptides. Orig. Life Evol. Biosph. 2017, 47, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Maeda, N.; Hayakawa, S. Chiral recognition between l-alanine peptides and tryptophan enantiomers probed by ultraviolet photodissociation in the gas phase. J. Mass Spectrom. 2016, 51, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, A.; Maeda, N.; Hayakawa, S. Enantioselective photolysis and quantitative chiral analysis of tryptophan complexed with alkali-metalized l-serine in the gas phase. Chirality 2015, 27, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Nanita, S.C.; Cooks, R.G. Serine octamers: Cluster formation, reactions, and implications for biomolecule homochirality. Angew. Chem. Int. Ed. 2006, 45, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Aribi, H.E.; Orlova, G.; Hopkinson, A.C.; Siu, K.W.M. Gas-phase fragmentation reactions of protonated aromatic amino acids: Concomitant and consecutive neutral eliminations and radical cation formations. J. Phys. Chem. A 2004, 108, 3844–3854. [Google Scholar] [CrossRef]
- Rivera-Tirado, E.; Wesdemiotis, C. Fragmentation characteristics of bn (n = 2–15) ions from protonated peptides. Rapid Commun. Mass Spectrom. 2011, 25, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Nishi, H.; Kamigauchi, M.; Ishida, T. Structural scaffold of 18-crown-6 tetracarboxylic acid for optical resolution of chiral amino acid: X-ray crystal analyses and energy calculation of complexes of d- and l-isomers of tyrosine, isoleucine, methionine and phenylglycine. Org. Biomol. Chem. 2004, 2, 3470–3475. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, H.H.L.; Ko, Y.H.; Kim, K.; Kim, H.I. Deciphering the specific high-affinity binding of cucurbit [7] uril to amino acids in water. J. Phys. Chem. B 2015, 119, 4628–4636. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, E.; Vilaseca, M.; Díaz-Lobo, M.; Masliy, A.N.; Vicent, C.; Fedin, V.P. Supramolecular adducts of cucurbit [7] uril and amino acids in the gas phase. J. Am. Soc. Mass Spectrom. 2016, 27, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, S.; Vandenbussche, G.; Reisse, J.; Bartik, K. Do serine octamers exist in solutions? Relevance of this question in the context of the origin of homochirality on Earth. Eur. J. Org. Chem. 2006, 3069–3073. [Google Scholar] [CrossRef]
- Schwartz, J.C.; Senko, M.W.; Syka, J.E.P. A two-dimensional quadrupole ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2002, 13, 659–669. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujihara, A.; Inoue, H.; Sogi, M.; Tajiri, M.; Wada, Y. Chiral and Molecular Recognition through Protonation between Aromatic Amino Acids and Tripeptides Probed by Collision-Activated Dissociation in the Gas Phase. Molecules 2018, 23, 162. https://doi.org/10.3390/molecules23010162
Fujihara A, Inoue H, Sogi M, Tajiri M, Wada Y. Chiral and Molecular Recognition through Protonation between Aromatic Amino Acids and Tripeptides Probed by Collision-Activated Dissociation in the Gas Phase. Molecules. 2018; 23(1):162. https://doi.org/10.3390/molecules23010162
Chicago/Turabian StyleFujihara, Akimasa, Hikaru Inoue, Masanobu Sogi, Michiko Tajiri, and Yoshinao Wada. 2018. "Chiral and Molecular Recognition through Protonation between Aromatic Amino Acids and Tripeptides Probed by Collision-Activated Dissociation in the Gas Phase" Molecules 23, no. 1: 162. https://doi.org/10.3390/molecules23010162
APA StyleFujihara, A., Inoue, H., Sogi, M., Tajiri, M., & Wada, Y. (2018). Chiral and Molecular Recognition through Protonation between Aromatic Amino Acids and Tripeptides Probed by Collision-Activated Dissociation in the Gas Phase. Molecules, 23(1), 162. https://doi.org/10.3390/molecules23010162