Free Radical-Scavenging Capacities, Phenolics and Capsaicinoids in Wild Piquin Chili (Capsicum annuum var. Glabriusculum)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content and Free Radical-Scavenging Capacity
2.2. Identification of Compounds by UPLC-ESI-Q/TOF-MSe
2.3. Capsaicinoids and SHU
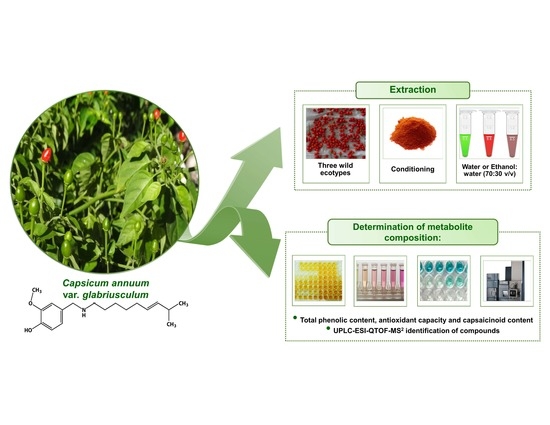
3. Materials and Methods
3.1. Plant Material and Sample Preparation
3.2. Methods of Extraction
3.3. TPC
3.4. Antioxidant Capacity
3.5. UPLC-ESI-Q/TOF-MSe Analysis
3.6. Capsaicinoid Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, V.S.P.; Prakash, I. The aroma, taste, color and bioactive constituents of tea. J. Med. Plants Res. 2011, 5, 2110–2124. [Google Scholar]
- Urbizu-González, A.L.; Castillo-Ruiz, O.; Martínez-Ávila, G.C.G.; Torres-Castillo, J.A. Natural variability of essential oil and antioxidants in the medicinal plant Turnera diffusa. Asian Pac. J. Trop. Biomed. 2017, 10, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Acunha, T.D.S.; Crizel, R.L.; Tavares, I.B.; Barbieri, R.L.; Pereira de Pereira, C.M.; Rombaldi, C.V.; Chaves, F.C. Bioactive Compound Variability in a Brazilian Pepper Collection. Crop. Sci. 2017, 57, 1–13. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Tikunov, Y.; de Vos, R.C.H.; Pelgrom, K.T.B.; Maharijaya, A.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolomics and molecular marker analysis to explore pepper (Capsicum sp.) biodiversity. Metabolomics 2013, 9, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Kang, Y.F.; Li, W.J.; Li, H.T.; Li, C.T.; Chen, C.Y. Secondary metabolites from the unripe fruits of Capsicum annuum var. conoides. Chem. Nat. Compd. 2016, 52, 1145–1146. [Google Scholar] [CrossRef]
- Meckelmann, S.W.; Riegel, D.W.; van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Capsaicinoids, flavonoids, tocopherols, antioxidant capacity and color attributes in 23 native Peruvian chili peppers (Capsicum spp.) grown in three different locations. Eur. Food Res. Technol. 2015, 240, 273–283. [Google Scholar] [CrossRef]
- González-Zamora, A.; Sierra-Campos, E.; Pérez-Morales, R.; Vázquez-Vázquez, C.; Gallegos-Robles, M.A.; López-Martínez, J.D.; García-Hernández, J.L. Measurement of capsaicinoids in Chiltepin hot pepper: A comparison study between spectrophotometric method and high-performance liquid chromatography analysis. J. Chem. 2015, 1–10. [Google Scholar] [CrossRef]
- Rochín-Wong, C.S.; Gámez-Meza, N.; Montoya-Ballesteros, L.C.; Medina-Juárez, L.A. Efecto de los procesos de secado y encurtido sobre la capacidad antioxidante de los fitoquímicos del chiltepín (Capsicum annuum L. var. glabriusculum). Rev. Mex. Ing. Quím. 2013, 12, 227–239. [Google Scholar]
- Rodríguez-Maturino, A.; Valenzuela-Solorio, A.; Troncoso-Rojas, R.; González-Mendoza, D.; Grimaldo-Juárez, O.; Avilés-Marín, M.; Cervantes-Diaz, L. Antioxidant activity and bioactive compounds of Chiltepin (Capsicum annuum var. glabriusculum) and Habanero (Capsicum chinense): A comparative study. J. Med. Plants Res. 2012, 6, 1758–1763. [Google Scholar] [CrossRef]
- Baenas, N.; Beovíc, M.; Llic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper. Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Schreiner, M. Vegetable crop management strategies to increase the quantity of phytochemicals. Eur. J. Nutr. 2005, 44, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Hernández, E.; San Miguel-Chávez, R.; Palma Tenango, M.; González-Trujano, M.E.; de la Rosa-Manzano, E.; Sánchez-Ramos, G.; Mora-Olivo, A.; Martínez-Palacios, A.; Martínez-Avalos, J.G. Capsaicinoids concentration in Capsicum annuum var. glabriusculum collected in Tamaulipas, Mexico. Phyton. Inter. J. Exp. Bot. 2017, 86, 46–52. [Google Scholar]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Vera-Guzmán, A.M.; Chávez-Servia, J.L.; Carrillo-Rodríguez, J.C.; López, M.G. Phytochemical evaluation of wild and cultivated pepper (Capsicum annuum L. and C. pubescens Ruiz & Pav.) from Oaxaca, Mexico. Chil. J. Agric. Res. 2011, 71, 578–585. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Pérez-Won, M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum L. var. Hungarian). Food Chem. 2009, 117, 647–653. [Google Scholar] [CrossRef]
- Kim, J.S.; Ahn, J.; Lee, S.J.; Moon, B.; Ha, T.Y.; Kim, S. Phytochemicals and antioxidant activity of fruits and leaves of paprika (Capsicum annuum L.; var. Special) cultivated in Korea. J. Food Sci. 2011, 76, C193–C198. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; De Luca, D.; O’Brien, N.; Menichini, F.; Tundis, R. Influence of drying and cooking process on the phytochemical content, antioxidant and hypoglycemic properties of two bell Capsicum annum L. cultivars. Food Chem. Toxicol. 2013, 53, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.A.; Menichini, F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007, 102, 1096–1104. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; García, O.P.; Rosado, J.L.; Goñi, I. The contribution of fruits and vegetables to dietary intake of polyphenols and antioxidant capacity in a Mexican rural diet: Importance of fruit and vegetable variety. Food Res. Int. 2011, 44, 1182–1189. [Google Scholar] [CrossRef]
- Wangcharoen, W.; Morasuk, W. Antioxidant capacity and phenolic content of chilies. Kasetsart J. (Nat. Sci.) 2007, 41, 561–569. [Google Scholar]
- Putnik, P.; Bursác Kovačevíc, D.; Ježek, D.; Šustić, I.; Zorić, Z.; Dragović-Uzelac, V. High-pressure recovery of anthocyanins from grape skin pomace (Vitis vinifera cv. Teran) at moderate temperature. J. Food Process. Preserv. 2018, 42, e13342. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jiang, T.; He, J.; Barba, F.J.; Cravotto, G.; Koubaa, M. Ultrasound-assisted extraction, centrifugation and ultrafiltration: Multistage process for polyphenol recovery from purple sweet potatoes. Molecules 2016, 21, 1584. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Zielinski, A.A.; Isidoro-Haminiuk, C.W.; Alberti, A.; Nogueira, A.; Mottin, D.I.; Granato, D.A. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res. Int. 2014, 60, 246–254. [Google Scholar] [CrossRef]
- Durak, A.; Kowalska, I.; Gawlik-Dziki, U. UPLC-MS method for determination of phenolic compounds in chili as a coffee supplement and their impact of phytochemicals interactions on antioxidant activity in vitro. Acta Chromatogr. 2017, 30, 1–6. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdylo, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, J.; Zhou, Q.; Lei, H.; Luan, L.; Liu, X.; Wu, Y. Indirect identification of antioxidants in Polygalae Radix through their reaction with 2,2-diphenyl-1-picrylhydrazyl and subsequent HPLC-ESI-Q-TOF-MS/MS. Talanta 2015, 144, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Vasileva, E.A.; Carne, A.; McConnell, M.; Bekhit, A.E.D.A.; Mishchenko, N.P. Naphthoquinones of the spinochrome class: Occurrence, isolation, biosynthesis and biomedical applications. RSC Adv. 2018, 8, 32637–32650. [Google Scholar] [CrossRef]
- Nakatani, N.; Kayano, S.I.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Comparison of in vitro antioxidant and antiradical activities of l-tyrosine and l-Dopa. Amino Acids 2007, 32, 431. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.H.; Mullen, W.; Crozier, A. Identification of proanthocyanidin dimers and trimers, flavone C-glycosides, and antioxidants in Ficus deltoidea, a Malaysian herbal tea. J. Agric. Food Chem. 2011, 59, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Morales-Soto, A.; Gómez-Caravaca, A.M.; García-Salas, P.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013, 51, 977–984. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 28 January 2017).
- Hurtado-Fernández, E.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Profiling LC-DAD-ESI-TOF MS Method for the Determination of Phenolic Metabolites from Avocado (Persea americana). J. Agric. Food Chem. 2011, 59, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Narodiya, V.P.; Vadodaria, M.S.; Vagadiya, G.V.; Gadara, S.A.; Ladwa, K.D. Synthesis, characterization and Biological Activiti Studies of substituted 6H-12Hbenzopyrano [4,3-b]quinolin-6-one. IJETR 2014, 2, 1–8. [Google Scholar]
- Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Simultaneous Determination of Phenolic Compounds and Saponins in Quinoa (Chenopodium quinoa Willd) by a Liquid Chromatography Diode Array Detection Electrospray Ionization Time-of-Flight Mass Spectrometry Methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.; Ferreres, F.; Tomás-Barberán, F.A.; Gil, M.I. Characterization and Quantitation of Antioxidant Constituents of Sweet Pepper (Capsicum annuum L.). J. Agric. Food Chem. 2004, 52, 3861–3869. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, Q.; Zhang, X.; He, B.; Xu, H.; Yin, Y.; Liu, R.; Liu, J.; Chen, X.; Bi, K. Simultaneous quantitation of polygalantoxanthone III and four ginsenoides by ultra-fast liquid chromatography with tandem mass spectrometry in rat and beagle dog plasm after oral administration of kai-Xin-San: Aplication to a comparative pharmacokinetic study. J. Sep. Sci. 2014, 37, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jaiswal, R.; Kuhnert, N. Analysis on different grades of highly-rated Jamaica Blue, Mountain coffees compared to easily available originated coffee beans. SCIREA J. Food 2016, 1, 15–27. [Google Scholar]
- López-Gutiérrez, N.; Romero-González, R.; Martínez-Vidal, J.L.; Garrido-Frenich, A. Determination of polyphenols in grape-based nutraceutical products using high resolution mass spectrometry. LWT-Food Sci. Technol. 2016, 71, 249–259. [Google Scholar] [CrossRef]
- Bhagawati, M.; Saikia, A. Cultivar variation for capsaicinoid content in some processed products of chilli. J. Hortic. Sci. 2015, 10, 210–215. [Google Scholar]
- Barbero, G.F.; Liazid, A.; Azaroual, L.; Palma, M.; Barroso, C.G. Capsaicinoid Contents in Peppers and Pepper-Related Spicy Foods. Int. J. Food Prop. 2016, 19, 485–493. [Google Scholar] [CrossRef]
- Gurung, T.; Techawongstien, S.; Suriharn, B.; Techawongstien, S. Stability analysis of yield and capsaicinoids content in chili (Capsicum spp.) grown across six environments. Euphytica 2012, 187, 11–18. [Google Scholar] [CrossRef]
- Votava, E.J.; Nabham, G.P.; Bosland, P.W. Genetic diversity and similarity revealed via molecular analysis among and within an in-situ population and ex situ accessions of chiltepin (Capsicum annuum var. glabriusculum). Conserv. Genet. 2002, 3, 123–129. [Google Scholar] [CrossRef]
- Valiente-Banuet, J.I.; Gutiérrez-Ochoa, A. Effect of Irrigation Frequency and Shade Levels on Vegetative Growth, Yield, and Fruit Quality of Piquin Pepper (Capsicum annuum L. var. glabriusculum). HortScience 2016, 51, 573–579. [Google Scholar]
- Hallmann, E.; Rembiałkowska, E. Characterization of antioxidant compounds in sweet bell pepper (Capsicum annuum L.) under organic and conventional growing systems. J. Sci. Food Agric. 2012, 92, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of different capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography, determination of pungency and effect of high temperature. Molecules 2013, 189. [Google Scholar] [CrossRef] [PubMed]
- Fricke, E.C.; Haak, D.C.; Levey, D.J.; Tewksbury, J.J. Gut passage and secondary metabolites alter the source of post-dispersal predation for bird-dispersed chili seeds. Oecologia 2016, 181, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.A. Capsicum and chilli. In Spice Crops; CABI Publishing International: New York, NY, USA, 2002; p. 190. [Google Scholar]
- Torres-Castillo, J.A.; Sinagawa-García, S.R.; Martínez-Ávila, G.C.G.; López-Flores, A.B.; Sánchez-González, E.I.; Aguirre-Arzola, V.E.; Torres-Acosta, R.I.; Olivares-Sáenz, E.; Osorio-Hernández, E.; Gutiérrez-Díez, A. Moringa oleifera: Phytochemical detection, antioxidants, enzymes and antifungal properties. Phyton. Int. J. Exp. Bot. 2013, 82, 193–202. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzimol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice, E.C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kumari, S.; Elancheran, R.; Kotoky, J.; Devi, R. Rapid screening and identification of phenolic antioxidants in Hydrocotyle sibthorpioides Lam. by UPLC–ESI-MS/MS. Food Chem. 2016, 203, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Wasmund, L.M.; Bosland, P.W. Improved method for quantifying capsaicinoids in Capsicum using high-performance liquid chromatography. Hortscience 1995, 30, 137–139. [Google Scholar]
- Topuz, A.; Ozdemir, F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J. Food Compos. Anal. 2007, 20, 596–602. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide: Statistics, version 9.3; Statistic Analysis System Institute: Cary, NC, USA, 2011. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Ecotype | Aqueous Extracts | Hydroalcoholic Extracts | ||||
|---|---|---|---|---|---|---|
| TPC | DPPH• | ABTS•+ | TPC | DPPH• | ABTS•+ | |
| I-1 | 188.5 ± 8.6 A | 29.8 ± 0.7 A | 95 ± 0.8 A | 272.3 ± 7.1 b | 57.9 ± 0.9 b | 125 ± 0.5 a |
| I-2 | 137.8 ± 10.2 B | 17.4 ± 2.3 C | 60.4 ± 1.1 B | 309.8 ± 7.9 a | 62.6 ± 3.3 a | 124.8 ± 0.7 a |
| I-3 | 129.8 ± 3.2 B | 22.8 ± 0.5 B | 40 ± 0.4 C | 252.8 ± 4.6 b | 53.2 ± 0.2 ab | 110 ± 5.7 b |
| I-4 | 127.2 ± 1.6 B | 22 ± 0.9 B | 39.8 ± 6.3 C | 263.7 ± 5.1 c | 55.6 ± 1.6 c | 115 ± 4.2 b |
| HSD | 18.1 | 3.4 | 8.4 | 16.5 | 5 | 9.3 |
| Population mean | 145.8 ± 26.7 | 23 ± 4.8 | 58.8 ± 23.6 | 274.6 ± 23 | 57.3 ± 4.0 | 118.7 ± 7.4 |
| II-1 | 271.9 ± 15.8 B | 1.1 ± 0.4 B | 124.7± 0.4 B | 515.2 ± 6.0 b | 82.5 ± 1.0 c | 287.5 ± 4.0 a |
| II-2 | 183.4 ± 3.0 C | 8.6 ± 0.6 A | 117.2 ± 1.9 C | 544.6 ± 1.1 a | 100 ± 0.3 a | 188.3 ± 6.5 c |
| II-3 | 353 ± 16.8 A | 1.4 ± 0.3 B | 189.7 ± 2.4 A | 434.4 ± 8.3 c | 88.6 ± 1.1 b | 263.7 ± 12.4 b |
| HSD | 33.7 | 1.2 | 4.5 | 14.9 | 2.1 | 21 |
| Population mean | 269.5 ± 74.4 | 3.7 ± 3.7 | 143.9 ± 34.6 | 498 ± 49.7 | 90.4 ± 7.7 | 246.5 ± 45.4 |
| III-1 | 174.3 ± 8.7 A | 8.4 ± 0.5 B | 117.2 ± 1.9 A | 497.9 ± 17.5 a | 90.3 ± 1.4 a | 283.9 ± 2.5 a |
| III-2 | 106.4 ± 1.1 B | 12.4 ± 1.0 A | 20.2 ± 1.7 B | 338.4 ± 17.9 b | 41.6 ± 0.7 b | 126 ± 0.8 b |
| HSD | 14 | 1.8 | 4.1 | 40.1 | 2.5 | 4.2 |
| Population mean | 140.3 ± 37.6 | 10.4 ± 2.3 | 68.7 ± 53.2 | 418.1 ± 88.8 | 66 ± 26.7 | 204.9 ± 86.5 |
| Peak N° | Rt (min) | [M − H]− (m/z) | Tentative Assignment | Molecular Formula | MS2 Dominant Fragments Ions | Compound Type | Ecotype (Population-Sample) | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-1 | I-2 | I-3 | I-4 | II-1 | II-2 | II-3 | III-1 | III-2 | ||||||||
| 1 | 0.846 | 191.0874 | Quinic acid | C7H11O6 | - | Phenolic acid | x | x | x | x | x | [34] | ||||
| 2 | 1.116 | 191.0492 | Citric acid | C6H8O7 | - | Organic acid | x | [34] | ||||||||
| 3 | 1.15 | 180.0985 | Tyrosine | C9H10NO3 | 163.0791 | Amino acid | x | x | x | x | x | x | x | [34] | ||
| 4 | 1.218 | 383.1263 | Kaempferol-7,4′-Dimethoxy-8-Butyryl ester | C21H20O7 | - | Flavonoid | x | x | x | x | [35] | |||||
| 5 | 1.252 | 312.0973 | 1,2,4-trihydroxynonadecane | C19H38O3 | 313.0905, 225.1853, 279.1969, 293.2083 | Fatty alcohol | x | x | x | x | [36] | |||||
| 6 | 1.319 | 169.0607 | Gallic acid | C7H6O5 | 125.0751 | Phenolic acid | x | * | ||||||||
| 7 | 1.421 | 282.0933 | Guanosine | C10H13N5O5 | 150.0444 | Nucleoside | x | x | x | x | x | x | [37] | |||
| 8 | 1.522 | 297.1259 | Genistein-4,7′-dimethyl ether | C17H14O5 | - | Flavonoid | x | x | [35] | |||||||
| 9 | 1.725 2.165 | 174.9907 | Ascorbic acid Isomer | C6H7O6 | - | Vitamin | x | x | x | [37] | ||||||
| 10 | 2.03 | 164.108 | dl-Phenylalanine | C9H10NO2 | 147.044 | Amino acid | x | x | x | x | x | x | [34] | |||
| 11 | 2.842 | 301.1075 | Quercetin | C15H10O7 | 173.1554 | Flavonoid | x | x | [38] | |||||||
| 12 | 2.909 | 233.1501 | Unknown | - | - | - | x | x | - | |||||||
| 13 | 3.282 | 203.1112 | Tryptophan | C11H11N2O2 | - | Amino acid | x | [34] | ||||||||
| 14 | 3.315 | 203.1091 | 7-Ethoxy-4-methylcoumarin | C12H12O3 | - | - | x | x | x | x | x | x | x | [35] | ||
| 15 | 3.349 3.484 | 310.1175 | Benzopyrano [4,3-β] quinoline-6-ones | C21H13NO2 | - | Alkaloid | x | [39] | ||||||||
| 16 | 3.417 | 329.086 | Vanillic acid 1-O-B-glucopyranosyl ester | C14H18O9 | - | Phenolic acid | x | x | x | x | x | x | x | x | [34] | |
| 17 | 3.518 | 331.1016 | 1-O-galloyl-β-d-glucose | C13H16O10 | - | Phenolic acid | x | [40] | ||||||||
| 18 | 3.552 3.721 | 311.0905 | 2-Caffeoyl-l-tartaric acid | C13H12O9 | 133.0135, 115.0034 | Phenolic acid | x | x | [38] | |||||||
| 19 | 3.958 | 337.1437 | Coumaroyl quinic acid I or II | C16H17O8 | 191.0571 | Phenolic acid | x | x | x | x | x | x | [37] | |||
| 20 | 4.161 | 263.1557 | Spinochrome A | C12H8O7 | 235.9501, 207.9648 | - | x | [38] | ||||||||
| 21 | 4.702 | 325.0885 | Coumaryl-hexoside | C15H18O8 | 163.0762 | Phenolic acid | x | x | x | x | x | x | [37] | |||
| 22 | 4.736 | 325.0894 | Coumaric acid hexose | C15H17O8 | 163.0762 | Phenolic acid | x | x | x | [37] | ||||||
| 23 | 4.838 | 457.1463 | 6′′-O-Acetyldaidzin | C23H21O10 | - | Flavonoid | x | [35] | ||||||||
| 24 | 4.872 | 457.1479 | Unknown | - | - | - | x | - | ||||||||
| 25 | 5.075 | 503.1013 | Quercetin-3-O-(6′′-O-acetyl)-β-d-glucopyranoside | C23H21O13 | - | Flavonoid | x | x | [35] | |||||||
| 26 | 5.075 | 653.0688 | Quercetin 3,7-diglucuronide | C27H26O19 | - | Flavonoid | x | [35] | ||||||||
| 27 | 5.108 5.446 | 563.0996 | Apigenin 6-C-hexoside-8-C-pentoside Isomer | C27H28O14 | 545.0913, 503.1136 | Flavonoid | x | [41] | ||||||||
| 28 | 5.244 | 355.0937 | Feruloyl-β-d-glucose | C16H20O9 | 175.0408 | Phenolic acid | x | x | x | x | x | x | [40] | |||
| 29 | 5.278 | 567.1243 | Polygala xanthone III | C25H28O15 | - | Xanthone | x | x | x | [42] | ||||||
| 30 | 5.379 | 352.0956 | 4-O-caffeoylquinic acid | C16H18O9 | 134.8 | Phenolic acid | x | x | x | x | x | x | [43] | |||
| 31 | 5.447 | 431.1585 | Apigenin 8-C-glucoside | C21H19O10 | 311.0561 | Flavonoid | x | x | x | x | x | x | x | x | x | [44] |
| 32 | 5.514 | 374.0412 | Unknown | - | - | - | x | x | x | x | x | - | ||||
| Ecotype (Population-Sample) | Capsaicin | Dihidrocapsaicin | Total Capsaicinoid Content | C:DHC | SHU |
|---|---|---|---|---|---|
| I-1 | 16.8 ± 0.2 b | 8.4 ± 0.6 a | 25.2 ± 0.7 a | 2.0:1 | 41,039.3 ± 1145.7 a |
| I-2 | 12.5 ± 0.1 d | 6.4 ± 0.05 b | 19.0 ± 0.1 c | 2.0:1 | 30,693.9 ± 232.2 c |
| I-3 | 18.6 ± 0.2 a | 8.6 ± 0.1 a | 27.2 ± 0.3 a | 2.2:1 | 44,034.9 ± 465.2 a |
| I-4 | 14.6 ± 0.3 c | 8.0 ± 0.2 a | 22.6 ± 0.5 b | 1.8:1 | 36,693.3 ± 874.5 b |
| Population mean | 15.6 | 7.9 | 23.5 | 38,115.3 | |
| HSD | 1.0 | 1.3 | 2.2 | 3469.6 | |
| CV% | 15.3 | 12.5 | 14.0 | 14.1 | |
| II-1 | 8.8 ± 0.1 b | 5.3 ± 0.05 b | 14.1 ± 0.1 b | 1.7:1 | 22,867.2 ± 227.6 b |
| II-2 | 9.7 ± 0.3 a | 6.7 ± 0.2 a | 16.4 ± 0.5 a | 1.4:1 | 26,460.6 ± 783.1 a |
| II-3 | 9.8 ± 0.05 a | 6.9 ± 0.03 a | 16.7 ± 0.1 a | 1.4:1 | 27,157.4 ± 38.7 a |
| Population mean | 11.5 | 6.3 | 15.7 | 25,495.1 | |
| HSD | 0.8 | 0.5 | 1.3 | 2045.2 | |
| CV% | 5.9 | 12.1 | 8.3 | 8.3 | |
| III-1 | 8.9 ± 0.05 b | 4.8 ± 0.01 b | 13.7 ± 0.1 b | 1.9:1 | 22,190.7 ± 111.3 b |
| III-2 | 14 ± 0.4 a | 5.6 ± 0.2 a | 19.5 ± 0.6 a | 2.5:1 | 31,972.1 ± 917.6 a |
| Population Mean | 9.4 | 5.2 | 16.6 | 27,081.4 | |
| HSD | 1.1 | 0.4 | 1.5 | 2566.3 | |
| CV% | 24.4 | 9.1 | 19.7 | 20.1 |
| Population | Ecotype (Population-Sample) | Place | Environment | |||
|---|---|---|---|---|---|---|
| Geographic Location (Decimal Grades) | Altitude (m) | Vegetation Type, Clime, Precipitation and Soil | ||||
| I | I-1 | Llera | 23.2781 N | −99.0681 W | 414 | Annual rainfed agriculture; Aw1; Precipitation in the driest month less than 60 mm; Vertisol. |
| I-2 | 23.2761 N | −99.0844 W | 403 | Secondary vegetation of low deciduous forest; Aw1; Precipitation in the driest month less than 60 mm; Regosol. | ||
| I-3 | 23.2825 N | −99.0635 W | 503 | Annual rainfed agriculture; (A) C (wo); Precipitation in the driest month less than 40 mm; Vertisol. | ||
| I-4 | 23.2824 N | −99.0624 W | 504 | |||
| II | II-1 | Hidalgo | 24.1258 N | −99.2569 W | 242 | Permanent irrigation agriculture; (A) C (wo); Precipitation in the driest month less than 40 mm; Vertisol. |
| II-2 | 24.1255 N | −99.2567 W | 249 | |||
| II-3 | 24.1143 N | −99.2496 W | 232 | |||
| III | III-1 | Soto La Marina | 23.7927 N | −98.1129 W | 30 | Annual irrigation agriculture; BS1 (h′) w; Summer showers; Rendzine. |
| III-2 | 23.8073 N | −98.0352 W | 60 | Cultivated pasture; (A) C (wo); Precipitation in the driest month of less than 40 mm; Litosol. | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Ramírez, Y.D.R.; Martínez-Ávila, G.C.G.; González-Hernández, V.A.; Castro-López, C.; Torres-Castillo, J.A. Free Radical-Scavenging Capacities, Phenolics and Capsaicinoids in Wild Piquin Chili (Capsicum annuum var. Glabriusculum). Molecules 2018, 23, 2655. https://doi.org/10.3390/molecules23102655
Moreno-Ramírez YDR, Martínez-Ávila GCG, González-Hernández VA, Castro-López C, Torres-Castillo JA. Free Radical-Scavenging Capacities, Phenolics and Capsaicinoids in Wild Piquin Chili (Capsicum annuum var. Glabriusculum). Molecules. 2018; 23(10):2655. https://doi.org/10.3390/molecules23102655
Chicago/Turabian StyleMoreno-Ramírez, Yolanda Del Rocio, Guillermo C. G. Martínez-Ávila, Víctor Arturo González-Hernández, Cecilia Castro-López, and Jorge Ariel Torres-Castillo. 2018. "Free Radical-Scavenging Capacities, Phenolics and Capsaicinoids in Wild Piquin Chili (Capsicum annuum var. Glabriusculum)" Molecules 23, no. 10: 2655. https://doi.org/10.3390/molecules23102655
APA StyleMoreno-Ramírez, Y. D. R., Martínez-Ávila, G. C. G., González-Hernández, V. A., Castro-López, C., & Torres-Castillo, J. A. (2018). Free Radical-Scavenging Capacities, Phenolics and Capsaicinoids in Wild Piquin Chili (Capsicum annuum var. Glabriusculum). Molecules, 23(10), 2655. https://doi.org/10.3390/molecules23102655