TrypanocidalActivity of Natural Sesquiterpenoids Involves Mitochondrial Dysfunction, ROS Production and Autophagic Phenotype in Trypanosoma cruzi
Abstract
:1. Introduction
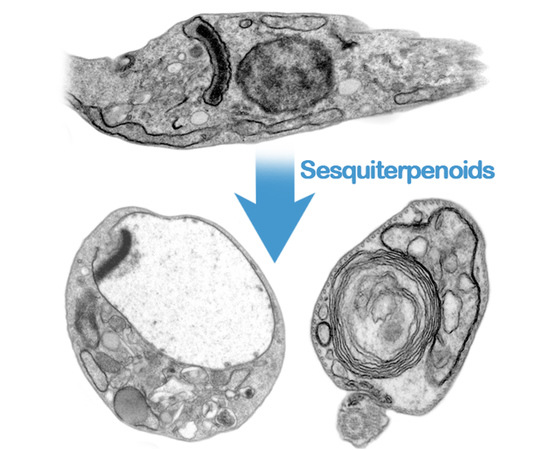
2. Results
3. Discussion
4. Materials and Methods
4.1. Purification and Chemical Analysis of Sesquiterpenoids
4.2. Animals and Parasites
4.3. Direct Effect on Bloodstream Trypomastigotes and Epimastigotes
4.4. Effect on Intracellular Amastigotes and Host Toxicity
4.5. Ultrastructural Analysis
4.6. Flow Cytometry Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Conteh, L.; Engels, T.; Molyneux, D.H. Socioeconomic aspects of neglected tropical diseases. Lancet 2010, 375, 239–247. [Google Scholar] [CrossRef]
- Bern, C.; Montgomery, S.P. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. 2009, 49, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Kjos, S.; Yabsley, M.J.; Montgomery, S.P. Trypanosoma cruzi and Chagas’ Disease in the United States. Clin. Microbiol. Rev. 2011, 24, 655–681. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Committee on the Control of Chagas Disease (Brasilia, Brazil) & World Health Organization. Control of Chagas Disease: Second Report of the WHO Expert Committee (Geneva). Available online: http://www.who.int/iris/handle/10665/42443 (accessed on 24 May 2018).
- Prata, A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 2001, 1, 92–100. [Google Scholar] [CrossRef]
- Soeiro, M.N.; de Castro, S.L. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin. Ther. Targets 2009, 13, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A. Specific chemotherapy of Chagas disease: Relevance, current limitations and new approaches. Acta Trop. 2010, 115, 55–68. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W. Special organelles of some pathogenic protozoa. Parasitol. Res. 2002, 88, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.C.; de Souza, W. Ultrastructural alterations in organelles of parasitic protozoa induced by different classes of metabolic inhibitors. Curr. Pharm. Des. 2008, 14, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, M.N.; de Castro, S.L. Screening of potential anti-Trypanosoma cruzi candidates: In vitro and in vivo studies. Open Med. Chem. J. 2011, 5, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.; de Castro, S.L. The double-edged sword in pathogenic trypanosomatids: The pivotal role of mitochondria in oxidative stress and bioenergetics. Biomed. Res. Int. 2014, 2014, 614014. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.; Salomão, K.; Dantas, A.P.; Santa-Rita, R.M.; Soares, M.J.; Barbosa, H.S.; de Castro, S.L. Different cell death pathways induced by drugs in Trypanosoma cruzi: An ultrastructural study. Micron 2009, 40, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.; Da Silva, E.N.; Pinto, A.V.; De Castro, S.L.; Menna-Barreto, R.F. A novel triazolic naphthofuranquinone induces autophagy in reservosomes and impairment of mitosis in Trypanosoma cruzi. Parasitology 2012, 139, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.S.; Castro, S.L. Between Armour and Weapons—Cell death mechanisms in trypanosomatid parasites. In Cell Death-Autophagy, Necrosis and Apoptosis, 1st ed.; Ntuli, T., Ed.; IntechOpen: Rijeka, Croatia, 2015; pp. 195–230. [Google Scholar]
- Coura, J.; de Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruzi. 2002, 97, 3–24. [Google Scholar] [CrossRef]
- Paz, C.; Cárcamo, G.; Silva, M.; Becerra, J.; Urrutia, H.; Sossa, K. Drimendiol, a drimane sesquiterpene with quorum sensing inhibition activity. Nat. Prod. Commun. 2013, 8, 147–148. [Google Scholar]
- Cárcamo, G.; Silva, M.; Becerra, J.; Urrutia, H.; Sossa, K.; Paz, C. Inhibition of quorum sensing by drimane lactones from chilean flora. J. Chilean Chem. Soc. 2014, 59, 2622–2624. [Google Scholar] [CrossRef]
- Hoeneisen, M.; Sicva, M.; Bohlmann, F. Sesquiterpene lactones of Podanthus mitiqui. Phytochemistry 1980, 19, 2765–2766. [Google Scholar] [CrossRef]
- Bautista, E.; Calzada, F.; Yepez-Mulia, L.; Chavez-Soto, M.; Ortega, A. IncomptinesC and D, two heliangolides from Decachaeta incompta and their antiprotozoal activity. Plant. Med. 2012, 78, 1698–1701. [Google Scholar]
- Blees, J.S.; Bokesch, H.R.; Rübsamen, D.; Schulz, K.; Milke, L.; Bajer, M.M.; Gustafson, K.R.; Henrich, C.J.; McMahon, J.B.; Colburn, N.H.; et al. Erioflorin stabilizes the tumor suppressor Pdcd4 by inhibiting its interaction with the E3-ligase β-TrCP1. PLoS ONE 2012, 7, e46567. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, M.; Wang, Q.; Holst, J.; White, J.M.; Hofmann, A.; Davis, R.A. Dihydro-β-agarofurans from the Australian endemic rainforest plant Denhamia pittosporoides inhibit leucine transport in prostate cancer cells. Asian J. Org. Chem. 2016, 5, 1461–1466. [Google Scholar] [CrossRef]
- Paz, C.; von Dossow, D.; Tiznado, V.; Suarez, S.; Cukiernik, F.D.; Baggio, R. A dihydro-[β]-agarofuransesquiterpene from Maytenus boaria. Acta Crystallogr. Sect. C 2017, 73, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, D.S.; Tempone, A.G.; Reimão, J.Q.; Taniwaki, N.N.; Romoff, P.; Fávero, O.A.; Sartorelli, P.; Mecchi, M.C.; Lago, J.H. Anti-leishmanial and anti-trypanosomal potential of polygodial isolated from stem barks of Drimys brasiliensis Miers (Winteraceae). Parasitol. Res. 2011, 109, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Herrera Acevedo, C.; Scotti, L.; FeitosaAlves, M.; FormigaMeloDiniz, M.F.; Scotti, M.T. Computer-aided drug design using sesquiterpene lactones as sources of new structures with potential activity against infectious neglected diseases. Molecules 2017, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.H.; Scotti, L.; Scotti, M.T. In silicostudies designed to select sesquiterpene lactones with potential antichagasic activity from an in-house Asteraceae database. Chem. Med. 2018, 13, 634–645. [Google Scholar]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal sesquiterpene lactones and other constituents from Tarchonanthus camphoratus and Schkuhria pinnata. J. Nat. Prod. 2018, 81, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Brunoro, G.V.; Caminha, M.A.; Ferreira, A.T.; Leprevost, F.V.; Carvalho, P.C.; Perales, J.; Valente, R.H.; Menna-Barreto, R.F. Reevaluating the Trypanosoma cruzi proteomic map: The shotgun description of bloodstream trypomastigotes. J. Proteom. 2015, 115, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Romanha, A.J.; Castro, S.L.; Soeiro, M.N.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zani, C.; et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruzi 2010, 105, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Reggiori, F.; Klionsky, D.J. Autophagy in the eukaryotic cell. Eukaryot. Cell 2002, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Brennand, A.; Gualdrón-López, M.; Coppens, I.; Rigden, D.J.; Ginger, M.L.; Michels, P.A. Autophagy in parasitic protists: Unique features and drug targets. Mol. Biochem. Parasitol. 2011, 177, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Duszenko, M.; Ginger, M.L.; Brennand, A.; Gualdrón-López, M.; Colombo, M.I.; Coombs, G.H.; Coppens, I.; Jayabalasingham, B.; Langsley, G.; de Castro, S.L.; et al. Autophagy in protists. Autophagy 2011, 7, 127–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menna-Barreto, R.F.; Goncalves, R.L.; Costa, E.M.; Silva, R.S.; Pinto, A.V.; Oliveira, M.F.; de Castro, S.L. The effects on Trypanosoma cruzi of novel synthetic naphthoquinones are mediated by mitochondrial dysfunction. Free Radic. Biol. Med. 2009, 47, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, D.O.; Sobral Alves, E.S.; Gonçalves, V.T.; Fontes, S.S.; Nogueira, M.L.; Suarez-Fontes, A.M.; Neves da Costa, J.B.; Rios-Santos, F.; Vannier-Santos, M.A. Effects of a novel β-lapachone derivative on Trypanosoma cruzi: Parasite death involving apoptosis, autophagy and necrosis. Int. J. Parasitol. Drugs Resist. 2016, 6, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.F.; Batista, D.a.G.; De Araújo, J.S.; Batista, M.M.; Lionel, J.; de Souza, E.M.; Hammer, E.R.; da Silva, P.B.; De Mieri, M.; Adams, M.; et al. Activities of psilostachyin A and cynaropicrin against Trypanosoma cruzi in vitro and in vivo. Antimicrob. Agents Chemother. 2013, 57, 5307–5314. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, T.; Klionsky, D.J. Eating the endoplasmic reticulum: Quality control by autophagy. Trends Cell Biol. 2007, 17, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Waller, R.F.; McConville, M.J. Developmental changes in lysosome morphology and function Leishmania parasites. Int. J. Parasitol. 2002, 32, 1435–1445. [Google Scholar] [CrossRef]
- Figueiredo, R.C.; Rosa, D.S.; Gomes, Y.M.; Nakasawa, M.; Soares, M.J. Reservosome: An endocytic compartment in epimastigote forms of the protozoan Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae). Correlation between endocytosis of nutrients and cell differentiation. Parasitology 2004, 129, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.; Corrêa, J.R.; Pinto, A.V.; Soares, M.J.; de Castro, S.L. Mitochondrial disruption and DNA fragmentation in Trypanosoma cruzi induced by naphthoimidazoles synthesized from beta-lapachone. Parasitol. Res. 2007, 101, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Sangenito, L.S.; Menna-Barreto, R.F.S.R.F.S.; Oliveira, A.C.; d’Avila-Levy, C.M.; Branquinha, M.H.; Santos, A.L.S. Primary evidence of the mechanisms of action of HIV aspartyl peptidase inhibitors on Trypanosoma cruzi trypomastigote forms. Int. J. Antimicrob. Agents 2018, 52, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Cuadrado, A.; Engedal, N.; Jirsova, Z.; Cahova, M. The role of free radicals in autophagy regulation: Implications for ageing. Oxid. Med. Cell Longev. 2018, 2018, 2450748. [Google Scholar] [CrossRef] [PubMed]
- Paz, C.; Burgos, B.; Suarez, S.; Baggio, R. Dendocarbin A: A sesquiterpene lactone from Drimys winteri. Acta Crystallogr. C Struct. Chem. 2014, 70, 1007–1010. [Google Scholar]
- Gonçalves, R.L.; Barreto, R.F.; Polycarpo, C.R.; Gadelha, F.R.; Castro, S.L.; Oliveira, M.F. A comparative assessment of mitochondrial function in epimastigotes and bloodstream trypomastigotes of Trypanosoma cruzi. J. Bioenerg. Biomembr. 2011, 43, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Simões-Silva, M.R.; Nefertiti, A.S.; De Araújo, J.S.; Batista, M.M.; Da Silva, P.B.; Bahia, M.T.; Menna-Barreto, R.S.; Pavão, B.P.; Green, J.; Farahat, A.A.; et al. Phenotypic screening in vitro of novel aromatic amidines against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2016, 60, 4701–4707. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Extract/Compound | IC50/24 h (μg/mL) | LC50/24 h (μg/mL) | SI c | ||
|---|---|---|---|---|---|
| Trypomastigotes | Epimastigotes | Amastigotes a,b | |||
| D. winteri TE d | 77.8 ± 2.3 e | 174.9 ± 23.2 | - f | - | - |
| Polygodial (Pgd) | 11.5 ± 2.1 | 84.4 ± 9.4 | 1.0 f ± 0.1 | 10.1 ± 2.3 | 10.3 |
| Cinnamolide | 196.3 ± 24.6 | >4000.0 | - | - | - |
| Dendocarbin A | >800.0 | >500.0 | - | - | - |
| Isodrimeninol | 50.5 ± 5.9 | 169.9 ± 19.6 | - | - | - |
| P. mitiqui TE (PmTE) | 5.6 ± 0.6 | 40.8 ± 5.3 | 1.0 ± 0.2 | 20.7 ± 0.6 | 21.5 |
| Erioflorin acetate (Efr) | 6.1 ± 1.6 | 55.5 ± 5.4 | 0.9 ± 0.1 | 24.9 ± 3.8 | 29.5 |
| M. boaria TE | >2500.0 | >1500.0 | - | - | - |
| MB-16 | >4000.0 | >2500.0 | - | - | - |
| MB-22 | >1000.0 | >4000.0 | - | - | - |
| Compound | Dose | Median | IV a |
|---|---|---|---|
| Control | - | 72.6 ± 24.6 b | 0.0 |
| Pgd | 85 μg/mL | 12.8 ± 2.8 * | −0.83 |
| 42 μg/mL | 14.6 ± 5.4 * | −0.80 | |
| 21 μg/mL | 55.9 ± 12.0 | −0.23 | |
| Efr | 50 μg/mL | 4.1 ± 0.6 * | −0.90 |
| 25 μg/mL | 8.4 ± 2.9 * | −0.88 | |
| 12 μg/mL | 58.6 ± 22.8 | −0.60 | |
| PmTE | 40 μg/mL | 7.4 ± 5.8 * | −0.94 |
| 20 μg/mL | 27.9 ± 5.2 * | −0.93 | |
| 10 μg/mL | 29.0 ± 20.4 * | −0.56 |
| Dose | Median | IV a | |
|---|---|---|---|
| Control | - | 10.8 ± 4.0 b | 0.0 |
| Menadione | 10 μM | 44.1 ± 4.9 * | 4.1 |
| Pgd | 85 μg/mL | 66.1 ± 6.6 * | 6.1 |
| 42 μg/mL | 63.4 ± 6.4 * | 5.9 | |
| 21 μg/mL | 66.8 ± 5.0 * | 6.2 | |
| Efr | 50 μg/mL | 38.8 ± 5.5 * | 4.7 |
| 25 μg/mL | 33.4 ± 3.5 * | 3.9 | |
| 12 μg/mL | 32.9 ± 4.1 * | 3.1 | |
| PmTE | 40 μg/mL | 50.6 ± 5.3 * | 3.6 |
| 20 μg/mL | 41.6 ± 12.3 * | 3.1 | |
| 10 μg/mL | 33.7 ± 6.8 * | 3.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bombaça, A.C.S.; Von Dossow, D.; Barbosa, J.M.C.; Paz, C.; Burgos, V.; Menna-Barreto, R.F.S. TrypanocidalActivity of Natural Sesquiterpenoids Involves Mitochondrial Dysfunction, ROS Production and Autophagic Phenotype in Trypanosoma cruzi. Molecules 2018, 23, 2800. https://doi.org/10.3390/molecules23112800
Bombaça ACS, Von Dossow D, Barbosa JMC, Paz C, Burgos V, Menna-Barreto RFS. TrypanocidalActivity of Natural Sesquiterpenoids Involves Mitochondrial Dysfunction, ROS Production and Autophagic Phenotype in Trypanosoma cruzi. Molecules. 2018; 23(11):2800. https://doi.org/10.3390/molecules23112800
Chicago/Turabian StyleBombaça, Ana Cristina Souza, Daniela Von Dossow, Juliana Magalhães Chaves Barbosa, Cristian Paz, Viviana Burgos, and Rubem Figueiredo Sadok Menna-Barreto. 2018. "TrypanocidalActivity of Natural Sesquiterpenoids Involves Mitochondrial Dysfunction, ROS Production and Autophagic Phenotype in Trypanosoma cruzi" Molecules 23, no. 11: 2800. https://doi.org/10.3390/molecules23112800
APA StyleBombaça, A. C. S., Von Dossow, D., Barbosa, J. M. C., Paz, C., Burgos, V., & Menna-Barreto, R. F. S. (2018). TrypanocidalActivity of Natural Sesquiterpenoids Involves Mitochondrial Dysfunction, ROS Production and Autophagic Phenotype in Trypanosoma cruzi. Molecules, 23(11), 2800. https://doi.org/10.3390/molecules23112800