Tuning Texture and Morphology of Mesoporous TiO2 by Non-Hydrolytic Sol-Gel Syntheses
Abstract
:1. Introduction
2. Results
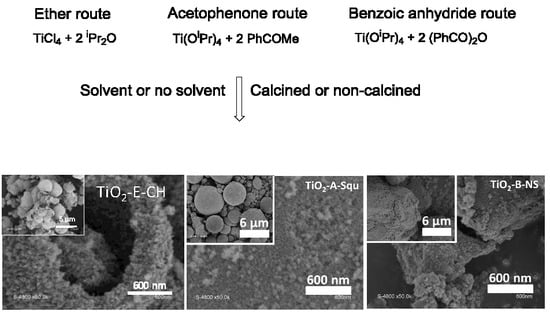
2.1. Synthesis of TiO2 Samples and Reactions Involved
2.2. Structural and Textural Characterization of TiO2 Samples
2.2.1. Powder X-Ray Diffraction (XRD)
2.2.2. Nitrogen Physisorption
2.3. Morphology of TiO2 Samples
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Park, G.C.; Seo, T.Y.; Jung, S.-B.; Lee, J.H.; Kim, Y.D.; Choi, D.H.; Lim, J.H.; Joo, J. Facet-controlled anatase TiO2 nanoparticles through various fluorine sources for superior photocatalytic activity. Nanotechnology 2016, 27, 395604. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent Progress on Titanium Dioxide Nanomaterials for Photocatalytic Applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Oi, L.E.; Choo, M.-Y.; Lee, H.V.; Ong, H.C.; Abd Hamid, S.B.; Juan, J.C. Recent advances of titanium dioxide (TiO2) for green organic synthesis. RSC Adv. 2016, 6, 108741–108754. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Mora-Seró, I.; De Angelis, F.; Bisquert, J.; Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Guo, H.; Liu, X.; Wu, S.; Yeung, K.W.K.; Chu, P.K. Nanostructured TiO2 for energy conversion and storage. RSC Adv. 2013, 3, 24758–24775. [Google Scholar] [CrossRef]
- Fröschl, T.; Hörmann, U.; Kubiak, P.; Kučerová, G.; Pfanzelt, M.; Weiss, C.K.; Behm, R.J.; Hüsing, N.; Kaiser, U.; Landfester, K.; Wohlfahrt-Mehrens, M. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Paik, U. TiO2 as an active or supplemental material for lithium batteries. J. Mater. Chem. A 2016, 4, 14–31. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclercq, D.; Lefèvre, P.; Mutin, P.H.; Vioux, A. Preparation of monolithic binary oxide gels by a non-hydrolytic sol-gel process. Chem. Mater. 1992, 4, 961–963. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclercq, D.; Lefèvre, P.; Mutin, P.H.; Vioux, A. Preparation of monolithic metal oxide gels by a non-hydrolytic sol–gel process. J. Mater. Chem. 1992, 2, 673–674. [Google Scholar] [CrossRef]
- Vioux, A. Nonhydrolytic Sol−Gel Routes to Oxides. Chem. Mater. 1997, 9, 2292–2299. [Google Scholar] [CrossRef]
- Pinna, N.; Niederberger, M. Surfactant-Free Nonaqueous Synthesis of Metal Oxide Nanostructures. Angew. Chem. Int. Ed. 2008, 47, 5292–5304. [Google Scholar] [CrossRef] [PubMed]
- Mutin, P.H.; Vioux, A. Nonhydrolytic processing of oxide-based materials: Simple routes to control homogeneity, morphology, and nanostructure. Chem. Mater. 2009, 21, 582–596. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Barnes, C.; Pinkas, J. The Power of Non-Hydrolytic Sol-Gel Chemistry: A Review. Catalysts 2017, 7, 168. [Google Scholar] [CrossRef]
- Deshmukh, R.; Niederberger, M. Mechanistic Aspects in the Formation, Growth and Surface Functionalization of Metal Oxide Nanoparticles in Organic Solvents. Chem.-Eur. J. 2017, 23, 8542–8570. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Hulea, V.; Mutin, P.H. Mesoporous mixed oxide catalysts via non-hydrolytic sol–gel: A review. Appl. Catal. A 2013, 451, 192–206. [Google Scholar] [CrossRef]
- Mutin, P.H.; Vioux, A. Recent advances in the synthesis of inorganic materials via non-hydrolytic condensation and related low-temperature routes. J. Mater. Chem. Coruña 2013, 1, 11504. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation of anatase, brookite and rutile at low temperature by non-hydrolytic sol–gel methods. J. Mater. Chem. 1996, 6, 1925–1932. [Google Scholar] [CrossRef]
- Popa, A.F.; Mutin, P.H.; Vioux, A.; Delahay, G.; Coq, B. Novel non-hydrolytic synthesis of a V2O5–TiO2 xerogel for the selective catalytic reduction of Nox by ammonia. Chem. Commun. 2004, 2214–2215. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Bouchmella, K.; Delaigle, R.; Eloy, P.; Poleunis, C.; Bertrand, P.; Gaigneaux, E.M.; Mutin, P.H. One-step non-hydrolytic sol–gel preparation of efficient V2O5-TiO2 catalysts for VOC total oxidation. Appl. Catal. B Environ. 2010, 94, 38–45. [Google Scholar] [CrossRef]
- Escamilla-Pérez, A.M.; Louvain, N.; Boury, B.; Brun, N.; Mutin, H. Ethers as oxygen donor and carbon source in non-hydrolytic sol-gel: One-pot, atom-economic synthesis of mesoporous TiO2-carbon nanocomposites. Chem. Eur. J. 2018, 24, 4982–4990. [Google Scholar] [CrossRef] [PubMed]
- Trentler, T.J.; Denler, T.E.; Bertone, J.F.; Agrawal, A.; Colvin, V.L. Synthesis of TiO2 Nanocrystals by Nonhydrolytic Solution-Based Reactions. J. Am. Chem. Soc. 1999, 121, 1613–1614. [Google Scholar] [CrossRef]
- Jun, Y.W.; Casula, M.F.; Sim, J.-H.; Kim, S.Y.; Cheon, J.; Alivisatos, A.P. Surfactant-Assisted Elimination of a High Energy Facet as a Means of Controlling the Shapes of TiO2 Nanocrystals. J. Am. Chem. Soc. 2003, 125, 15981–15985. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Park, J.; Kim, Y.; Choi, S.-H.; Sung, Y.-E.; Hyeon, T. Simultaneous phase- and size-controlled synthesis of TiO2 nanorods via non-hydrolytic sol−gel reaction of syringe pump delivered precursors. J. Phys. Chem. B 2006, 110, 24318–24323. [Google Scholar] [CrossRef] [PubMed]
- Aboulaich, A.; Boury, B.; Mutin, P.H. Reactive and organosoluble anatase nanoparticles by a surfactant-free nonhydrolytic synthesis. Chem. Mater. 2010, 22, 4519–4521. [Google Scholar] [CrossRef]
- Lou, F.; Qian, X.; Jin, Y.; Zhou, M. Characterisation of water-soluble TiO2 and its photocatalytic activity under visible light. Mater. Res. Innov. 2015, 19, S8-693–S8-696. [Google Scholar] [CrossRef]
- Niederberger, M.; Bartl, M.H.; Stucky, G.D. Benzyl alcohol and titanium tetrachloridea versatile reaction system for the nonaqueous and low-temperature preparation of crystalline and luminescent titania nanoparticles. Chem. Mater. 2002, 14, 4364–4370. [Google Scholar] [CrossRef]
- Garnweitner, G.; Niederberger, M. Nonaqueous and Surfactant-Free Synthesis Routes to Metal Oxide Nanoparticles. J. Am. Ceram. Soc. 2006, 89, 1801–1808. [Google Scholar] [CrossRef]
- Pinna, N.; Karmaoui, M.; Willinger, M.-G. The “benzyl alcohol route”: An elegant approach towards doped and multimetal oxide nanocrystals. J. Sol-Gel Sci. Technol. 2011, 57, 323–329. [Google Scholar] [CrossRef]
- Garnweitner, G.; Antonietti, M.; Niederberger, M. Nonaqueous synthesis of crystalline anatase nanoparticles in simple ketones and aldehydes as oxygen-supplying agents. Chem. Commun. 2005, 397. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.-M.; Xu, B.-Q. Solvothermal synthesis of TiO2: Anatase nanocrystals and rutile nanofibres from ticl4 in acetone. Appl. Organomet. Chem. 2007, 21, 146–149. [Google Scholar] [CrossRef]
- Pazik, R.; Tekoriute, R.; Håkansson, S.; Wiglusz, R.; Strek, W.; Seisenbaeva, G.A.; Gun’ko, Y.K.; Kessler, V.G. Precursor and solvent effects in the nonhydrolytic synthesis of complex oxide nanoparticles for bioimaging applications by the ether elimination (bradley) reaction. Chem.-Eur. J. 2009, 15, 6820–6826. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







| Sample | Precursor (amount) | O-donor a | Solvent | Temperature (time) | Calc. yield b/% |
|---|---|---|---|---|---|
| TiO2-E-NS | TiCl4 (12.5 mmol) | iPr2O | none | 110 °C (72 h) | 93 |
| TiO2-E-Tol | TiCl4 (12.5 mmol) | iPr2O | toluene (5 mL) | 110 °C (72 h) | 94 |
| TiO2-E-Squ | TiCl4 (12.5 mmol) | iPr2O | squalane (5 mL) | 110 °C (72 h) | 96 |
| TiO2-E-CH | TiCl4 (12.5 mmol) | iPr2O | cyclohexane (5 mL) | 110 °C (72 h) | 93 |
| TiO2-A-NS | Ti(OiPr)4 (4.4 mmol) | PhCOCH3 | none | 200 °C (12 h) | 92 |
| TiO2-A-Tol | Ti(OiPr)4 (4.4 mmol) | PhCOCH3 | toluene (10 mL) | 200 °C (12 h) | 95 |
| TiO2-A-Squ | Ti(OiPr)4 (4.4 mmol) | PhCOCH3 | squalane (10 mL) | 200 °C (12 h) | 96 |
| TiO2-B-NS | Ti(OiPr)4 (4.4 mmol) | (PhCO)2O | none | 200 °C (12 h) | 56 |
| TiO2-B-Tol | Ti(OiPr)4 (4.4 mmol) | (PhCO)2O | toluene (10 mL) | 200 °C (12 h) | 53 |
| TiO2-B-Squ | Ti(OiPr)4 (4.4 mmol) | (PhCO)2O | squalane (10 mL) | 200 °C (12 h) | 78 |
| Sample | Calcination | Cryst. size a (nm) | SBET b (m2 g−1) | Vp c (cm3 g−1) | Vmeso d (cm3 g−1) | Dp e (nm) |
|---|---|---|---|---|---|---|
| TiO2-E-NS | no yes | 9 23 | 180 70 | 0.28 0.19 | 0.27 0.18 | 5 9 |
| TiO2-E-Tol | no yes | 8 28 | 130 60 | 0.10 0.15 | 0.08 0.15 | 4 7 |
| TiO2-E-Squ | no yes | 8 17 | 170 80 | 0.31 0.24 | 0.31 0.23 | 7 10 |
| TiO2-E-CH | no yes | 13 28 | 130 50 | 0.18 0.14 | 0.17 0.14 | 5 9 |
| TiO2-A-NS | no yes | 11 12 | 90 70 | 0.32 0.29 | 0.32 0.28 | 10 12 |
| TiO2-A-Tol | no yes | 9 9 | 120 110 | 0.35 0.35 | 0.35 0.35 | 10 11 |
| TiO2-A-Squ | no yes | 12 13 | 120 100 | 0.48 0.35 | 0.48 0.34 | 13 10 |
| TiO2-B-NS | no yes | 9 12 | 240 80 | 0.13 0.15 | 0.02 0.15 | 3 6 |
| TiO2-B-Tol | no yes | am. 13 | 250 <10 | 0.14 <0.05 | 0.03 NA | 3 NA |
| TiO2-B-Squ | no yes | 7 14 | 240 90 | 0.16 0.42 | 0.07 0.42 | 4 15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bouchneb, M.; Alauzun, J.G.; Mutin, P.H. Tuning Texture and Morphology of Mesoporous TiO2 by Non-Hydrolytic Sol-Gel Syntheses. Molecules 2018, 23, 3006. https://doi.org/10.3390/molecules23113006
Wang Y, Bouchneb M, Alauzun JG, Mutin PH. Tuning Texture and Morphology of Mesoporous TiO2 by Non-Hydrolytic Sol-Gel Syntheses. Molecules. 2018; 23(11):3006. https://doi.org/10.3390/molecules23113006
Chicago/Turabian StyleWang, Yanhui, Maroua Bouchneb, Johan G. Alauzun, and P. Hubert Mutin. 2018. "Tuning Texture and Morphology of Mesoporous TiO2 by Non-Hydrolytic Sol-Gel Syntheses" Molecules 23, no. 11: 3006. https://doi.org/10.3390/molecules23113006
APA StyleWang, Y., Bouchneb, M., Alauzun, J. G., & Mutin, P. H. (2018). Tuning Texture and Morphology of Mesoporous TiO2 by Non-Hydrolytic Sol-Gel Syntheses. Molecules, 23(11), 3006. https://doi.org/10.3390/molecules23113006